[English] 日本語
 Yorodumi
Yorodumi- EMDB-15866: Cryo-EM structure of succinate dehydrogenase complex (complex-II)... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of succinate dehydrogenase complex (complex-II) in respiratory supercomplex of Tetrahymena thermophila | |||||||||||||||
 Map data Map data | Sharpened mask refined map | |||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Ciliate / Mitochondrial / Complex-II / Supercomplex / ELECTRON TRANSPORT | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationmitochondrial electron transport, succinate to ubiquinone / succinate dehydrogenase (quinone) activity / succinate dehydrogenase / 3 iron, 4 sulfur cluster binding / tricarboxylic acid cycle / respiratory electron transport chain / 2 iron, 2 sulfur cluster binding / flavin adenine dinucleotide binding / 4 iron, 4 sulfur cluster binding / electron transfer activity ...mitochondrial electron transport, succinate to ubiquinone / succinate dehydrogenase (quinone) activity / succinate dehydrogenase / 3 iron, 4 sulfur cluster binding / tricarboxylic acid cycle / respiratory electron transport chain / 2 iron, 2 sulfur cluster binding / flavin adenine dinucleotide binding / 4 iron, 4 sulfur cluster binding / electron transfer activity / mitochondrial inner membrane / metal ion binding / membrane Similarity search - Function | |||||||||||||||
| Biological species |  | |||||||||||||||
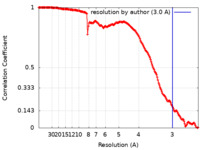
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||||||||
 Authors Authors | Muhleip A / Kock Flygaard R / Baradaran R / Amunts A | |||||||||||||||
| Funding support |  Sweden, European Union, 4 items Sweden, European Union, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Structural basis of mitochondrial membrane bending by the I-II-III-IV supercomplex. Authors: Alexander Mühleip / Rasmus Kock Flygaard / Rozbeh Baradaran / Outi Haapanen / Thomas Gruhl / Victor Tobiasson / Amandine Maréchal / Vivek Sharma / Alexey Amunts /      Abstract: Mitochondrial energy conversion requires an intricate architecture of the inner mitochondrial membrane. Here we show that a supercomplex containing all four respiratory chain components contributes ...Mitochondrial energy conversion requires an intricate architecture of the inner mitochondrial membrane. Here we show that a supercomplex containing all four respiratory chain components contributes to membrane curvature induction in ciliates. We report cryo-electron microscopy and cryo-tomography structures of the supercomplex that comprises 150 different proteins and 311 bound lipids, forming a stable 5.8-MDa assembly. Owing to subunit acquisition and extension, complex I associates with a complex IV dimer, generating a wedge-shaped gap that serves as a binding site for complex II. Together with a tilted complex III dimer association, it results in a curved membrane region. Using molecular dynamics simulations, we demonstrate that the divergent supercomplex actively contributes to the membrane curvature induction and tubulation of cristae. Our findings highlight how the evolution of protein subunits of respiratory complexes has led to the I-II-III-IV supercomplex that contributes to the shaping of the bioenergetic membrane, thereby enabling its functional specialization. #1:  Journal: Biorxiv / Year: 2022 Journal: Biorxiv / Year: 2022Title: Structural basis of mitochondrial membrane bending by I-II-III2-IV2 supercomplex Authors: Muhleip A / Flygaard RK / Haapanen O / Baradaran R / Gruhl T / Tobiasson V / Marechal A / Sharma V / Amunts A | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15866.map.gz emd_15866.map.gz | 398.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15866-v30.xml emd-15866-v30.xml emd-15866.xml emd-15866.xml | 35.9 KB 35.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15866_fsc.xml emd_15866_fsc.xml | 16.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_15866.png emd_15866.png | 62.8 KB | ||
| Masks |  emd_15866_msk_1.map emd_15866_msk_1.map | 421.9 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-15866.cif.gz emd-15866.cif.gz | 8.6 KB | ||
| Others |  emd_15866_additional_1.map.gz emd_15866_additional_1.map.gz emd_15866_half_map_1.map.gz emd_15866_half_map_1.map.gz emd_15866_half_map_2.map.gz emd_15866_half_map_2.map.gz | 213 MB 390.9 MB 390.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15866 http://ftp.pdbj.org/pub/emdb/structures/EMD-15866 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15866 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15866 | HTTPS FTP |
-Related structure data
| Related structure data |  8b6gMC  8b6fC  8b6hC  8b6jC  8bqsC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_15866.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15866.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened mask refined map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.25 Å | ||||||||||||||||||||||||||||||||||||
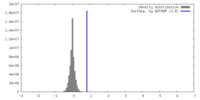
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_15866_msk_1.map emd_15866_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
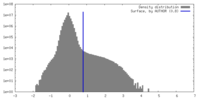
| Density Histograms |
-Additional map: Unsharpened mask refined map
| File | emd_15866_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened mask refined map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half-map A
| File | emd_15866_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half-map B
| File | emd_15866_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Succinate dehydrogenase complex (complex-II)
+Supramolecule #1: Succinate dehydrogenase complex (complex-II)
+Macromolecule #1: Diphthamide synthesis protein
+Macromolecule #2: Transmembrane protein, putative
+Macromolecule #3: Transposase
+Macromolecule #4: Succinate dehydrogenase [ubiquinone] flavoprotein subunit, mitoch...
+Macromolecule #5: DUF4885 domain-containing protein
+Macromolecule #6: Succinate dehydrogenase (quinone)
+Macromolecule #7: Transmembrane protein, putative
+Macromolecule #8: SDHTT3
+Macromolecule #9: Transmembrane protein, putative
+Macromolecule #10: NmrA domain-containing protein
+Macromolecule #11: Transmembrane protein, putative
+Macromolecule #12: Transmembrane protein, putative
+Macromolecule #13: Cytochrome b-c1 complex subunit 8
+Macromolecule #14: SDHTT11
+Macromolecule #15: SDHD
+Macromolecule #16: CARDIOLIPIN
+Macromolecule #17: CALCIUM ION
+Macromolecule #18: FLAVIN-ADENINE DINUCLEOTIDE
+Macromolecule #19: FE2/S2 (INORGANIC) CLUSTER
+Macromolecule #20: IRON/SULFUR CLUSTER
+Macromolecule #21: FE3-S4 CLUSTER
+Macromolecule #22: 1,2-Distearoyl-sn-glycerophosphoethanolamine
+Macromolecule #23: HEME C
+Macromolecule #24: 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE
+Macromolecule #25: Ubiquinone-8
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Average electron dose: 25.66 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.6 µm / Nominal defocus min: 0.6 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)