[English] 日本語
 Yorodumi
Yorodumi- EMDB-12665: Cryo-EM structure of pre-dephosphorylation complex of phosphoryla... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12665 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of pre-dephosphorylation complex of phosphorylated eIF2alpha with trapped holophosphatase (PP1A_D64A/PPP1R15A/G-actin/DNase I) | |||||||||
 Map data Map data | Unsharpened GS-FSC map after corrected FSC-mask auto-tightening from non-uniform refinement in CryoSPARC. The contour level was set when opend in UCSF Chimera. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | holophosphatase / PP1 / PPP1R15A / phosphorylated eIF2alpha / HYDROLASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationfatty acid derivative binding / positive regulation of translational initiation in response to stress / regulation of neutrophil mediated cytotoxicity / zymogen granule / regulation of acute inflammatory response / eukaryotic initiation factor eIF2 binding / translation initiation ternary complex / regulation of translation in response to endoplasmic reticulum stress / glial limiting end-foot / HRI-mediated signaling ...fatty acid derivative binding / positive regulation of translational initiation in response to stress / regulation of neutrophil mediated cytotoxicity / zymogen granule / regulation of acute inflammatory response / eukaryotic initiation factor eIF2 binding / translation initiation ternary complex / regulation of translation in response to endoplasmic reticulum stress / glial limiting end-foot / HRI-mediated signaling / deoxyribonuclease I / response to manganese-induced endoplasmic reticulum stress / PTW/PP1 phosphatase complex / Cellular response to mitochondrial stress / positive regulation of type B pancreatic cell apoptotic process / Response of EIF2AK1 (HRI) to heme deficiency / Recycling of eIF2:GDP / negative regulation of translational initiation in response to stress / PERK-mediated unfolded protein response / protein phosphatase type 1 complex / PERK regulates gene expression / RNA polymerase II promoter clearance / response to kainic acid / RNA polymerase II CTD heptapeptide repeat S5 phosphatase activity / deoxyribonuclease I activity / eukaryotic translation initiation factor 2 complex / neutrophil activation involved in immune response / protein localization to endoplasmic reticulum / protein phosphatase 1 binding / regulation of translational initiation in response to stress / protein phosphatase regulator activity / eukaryotic 48S preinitiation complex / DNA catabolic process / regulation of translational initiation / cytoskeletal motor activator activity / myosin heavy chain binding / glycogen metabolic process / Formation of the ternary complex, and subsequently, the 43S complex / protein dephosphorylation / entrainment of circadian clock by photoperiod / protein-serine/threonine phosphatase / tropomyosin binding / detection of maltose stimulus / actin filament bundle / negative regulation of PERK-mediated unfolded protein response / Ribosomal scanning and start codon recognition / troponin I binding / maltose transport complex / filamentous actin / mesenchyme migration / Translation initiation complex formation / protein serine/threonine phosphatase activity / carbohydrate transport / phosphatase activity / skeletal muscle myofibril / actin filament bundle assembly / striated muscle thin filament / negative regulation of transcription elongation by RNA polymerase II / skeletal muscle thin filament assembly / protein phosphatase activator activity / actin monomer binding / transition metal ion binding / intrinsic apoptotic signaling pathway in response to endoplasmic reticulum stress / carbohydrate transmembrane transporter activity / maltose binding / maltose transport / Response of EIF2AK4 (GCN2) to amino acid deficiency / maltodextrin transmembrane transport / positive regulation of signal transduction by p53 class mediator / GTP hydrolysis and joining of the 60S ribosomal subunit / L13a-mediated translational silencing of Ceruloplasmin expression / mitophagy / ATP-binding cassette (ABC) transporter complex, substrate-binding subunit-containing / skeletal muscle fiber development / stress fiber / titin binding / phosphoprotein phosphatase activity / actin filament polymerization / translation initiation factor activity / ATP-binding cassette (ABC) transporter complex / stress granule assembly / cellular response to amino acid starvation / response to endoplasmic reticulum stress / Downregulation of TGF-beta receptor signaling / cell chemotaxis / actin filament / filopodium / circadian regulation of gene expression / positive regulation of transcription elongation by RNA polymerase II / translational initiation / PKR-mediated signaling / regulation of circadian rhythm / ABC-family proteins mediated transport / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / cytoplasmic stress granule / calcium-dependent protein binding / cellular response to UV / nuclear envelope / positive regulation of canonical Wnt signaling pathway / lamellipodium Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /    | |||||||||
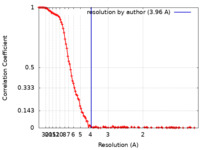
| Method | single particle reconstruction / cryo EM / Resolution: 3.96 Å | |||||||||
 Authors Authors | Yan Y / Hardwick S | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2021 Journal: Nat Struct Mol Biol / Year: 2021Title: Higher-order phosphatase-substrate contacts terminate the integrated stress response. Authors: Yahui Yan / Heather P Harding / David Ron /  Abstract: Many regulatory PPP1R subunits join few catalytic PP1c subunits to mediate phosphoserine and phosphothreonine dephosphorylation in metazoans. Regulatory subunits engage the surface of PP1c, locally ...Many regulatory PPP1R subunits join few catalytic PP1c subunits to mediate phosphoserine and phosphothreonine dephosphorylation in metazoans. Regulatory subunits engage the surface of PP1c, locally affecting flexible access of the phosphopeptide to the active site. However, catalytic efficiency of holophosphatases towards their phosphoprotein substrates remains unexplained. Here we present a cryo-EM structure of the tripartite PP1c-PPP1R15A-G-actin holophosphatase that terminates signaling in the mammalian integrated stress response (ISR) in the pre-dephosphorylation complex with its substrate, translation initiation factor 2α (eIF2α). G-actin, whose essential role in eIF2α dephosphorylation is supported crystallographically, biochemically and genetically, aligns the catalytic and regulatory subunits, creating a composite surface that engages the N-terminal domain of eIF2α to position the distant phosphoserine-51 at the active site. Substrate residues that mediate affinity for the holophosphatase also make critical contacts with eIF2α kinases. Thus, a convergent process of higher-order substrate recognition specifies functionally antagonistic phosphorylation and dephosphorylation in the ISR. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12665.map.gz emd_12665.map.gz | 73.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12665-v30.xml emd-12665-v30.xml emd-12665.xml emd-12665.xml | 28.2 KB 28.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12665_fsc.xml emd_12665_fsc.xml | 15.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_12665.png emd_12665.png | 205.4 KB | ||
| Masks |  emd_12665_msk_1.map emd_12665_msk_1.map | 149.9 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-12665.cif.gz emd-12665.cif.gz | 8.3 KB | ||
| Others |  emd_12665_additional_1.map.gz emd_12665_additional_1.map.gz emd_12665_half_map_1.map.gz emd_12665_half_map_1.map.gz emd_12665_half_map_2.map.gz emd_12665_half_map_2.map.gz | 13.4 MB 139 MB 139 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12665 http://ftp.pdbj.org/pub/emdb/structures/EMD-12665 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12665 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12665 | HTTPS FTP |
-Related structure data
| Related structure data |  7nzmMC  7nxvC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12665.map.gz / Format: CCP4 / Size: 149.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12665.map.gz / Format: CCP4 / Size: 149.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened GS-FSC map after corrected FSC-mask auto-tightening from non-uniform refinement in CryoSPARC. The contour level was set when opend in UCSF Chimera. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
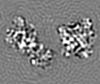
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.652 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
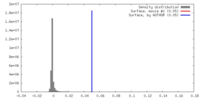
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_12665_msk_1.map emd_12665_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
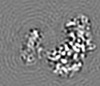
| Projections & Slices |
| ||||||||||||
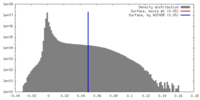
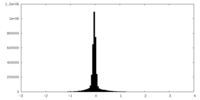
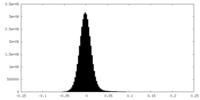
| Density Histograms |
-Additional map: ResolveCyroEM map; 1.81rmsd (0.443e/A^3) in COOT
| File | emd_12665_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ResolveCyroEM map; 1.81rmsd (0.443e/A^3) in COOT | ||||||||||||
| Projections & Slices |
| ||||||||||||
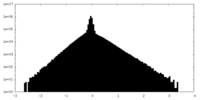
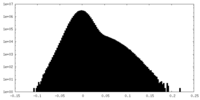
| Density Histograms |
-Half map: half (A) map of the main map
| File | emd_12665_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half (A) map of the main map | ||||||||||||
| Projections & Slices |
| ||||||||||||
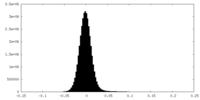
| Density Histograms |
-Half map: half (B) map of the main map
| File | emd_12665_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half (B) map of the main map | ||||||||||||
| Projections & Slices |
| ||||||||||||
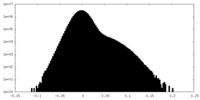
| Density Histograms |
- Sample components
Sample components
-Entire : pre-dephosphorylation complex of phosphorylated eIF2alpha_2-187 w...
| Entire | Name: pre-dephosphorylation complex of phosphorylated eIF2alpha_2-187 with trapped holophosphatase (PP1A_D64A/PPP1R15A_553-624/G-actin) |
|---|---|
| Components |
|
-Supramolecule #1: pre-dephosphorylation complex of phosphorylated eIF2alpha_2-187 w...
| Supramolecule | Name: pre-dephosphorylation complex of phosphorylated eIF2alpha_2-187 with trapped holophosphatase (PP1A_D64A/PPP1R15A_553-624/G-actin) type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 Details: One copy of each component was present in the complex: phosphorylated eIF2alpha_2-187, PP1A_D64A, PPP1R15A_553-624, G-actin and DNase I. The full complex was purified by size exclusion chromatography. |
|---|---|
| Molecular weight | Theoretical: 181 KDa |
-Macromolecule #1: Eukaryotic translation initiation factor 2 subunit 1
| Macromolecule | Name: Eukaryotic translation initiation factor 2 subunit 1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 21.817863 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: PGLSCRFYQH KFPEVEDVVM VNVRSIAEMG AYVSLLEYNN IEGMILLSEL (SEP)RRRIRSINK LIRIGRNECV VVIRVD KEK GYIDLSKRRV SPEEAIKCED KFTKSKTVYS ILRHVAEVLE YTKDEQLESL FQRTAWVFDD KYKRPGYGAY DAFKHAV SD PSILDSLDLN EDEREVLINN INRRLTPQ UniProtKB: Eukaryotic translation initiation factor 2 subunit 1 |
-Macromolecule #2: Serine/threonine-protein phosphatase PP1-alpha catalytic subunit
| Macromolecule | Name: Serine/threonine-protein phosphatase PP1-alpha catalytic subunit type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: protein-serine/threonine phosphatase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 33.647621 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: LNLDSIIGRL LEVQGSRPGK NVQLTENEIR GLCLKSREIF LSQPILLELE APLKICGAIH GQYYDLLRLF EYGGFPPESN YLFLGDYVD RGKQSLETIC LLLAYKIKYP ENFFLLRGNH ECASINRIYG FYDECKRRYN IKLWKTFTDC FNCLPIAAIV D EKIFCCHG ...String: LNLDSIIGRL LEVQGSRPGK NVQLTENEIR GLCLKSREIF LSQPILLELE APLKICGAIH GQYYDLLRLF EYGGFPPESN YLFLGDYVD RGKQSLETIC LLLAYKIKYP ENFFLLRGNH ECASINRIYG FYDECKRRYN IKLWKTFTDC FNCLPIAAIV D EKIFCCHG GLSPDLQSME QIRRIMRPTD VPDQGLLCDL LWSDPDKDVQ GWGENDRGVS FTFGAEVVAK FLHKHDLDLI CR AHQVVED GYEFFAKRQL VTLFSAPNYC GEFDNAGAMM SVDETLMCSF QILKPAD UniProtKB: Serine/threonine-protein phosphatase PP1-alpha catalytic subunit |
-Macromolecule #3: Actin, alpha skeletal muscle, intermediate form
| Macromolecule | Name: Actin, alpha skeletal muscle, intermediate form / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 41.862613 KDa |
| Sequence | String: DEDETTALVC DNGSGLVKAG FAGDDAPRAV FPSIVGRPRH QGVMVGMGQK DSYVGDEAQS KRGILTLKYP IEHGIITNWD DMEKIWHHT FYNELRVAPE EHPTLLTEAP LNPKANREKM TQIMFETFNV PAMYVAIQAV LSLYASGRTT GIVLDSGDGV T HNVPIYEG ...String: DEDETTALVC DNGSGLVKAG FAGDDAPRAV FPSIVGRPRH QGVMVGMGQK DSYVGDEAQS KRGILTLKYP IEHGIITNWD DMEKIWHHT FYNELRVAPE EHPTLLTEAP LNPKANREKM TQIMFETFNV PAMYVAIQAV LSLYASGRTT GIVLDSGDGV T HNVPIYEG YALPHAIMRL DLAGRDLTDY LMKILTERGY SFVTTAEREI VRDIKEKLCY VALDFENEMA TAASSSSLEK SY ELPDGQV ITIGNERFRC PETLFQPSFI GMESAGIHET TYNSIMKCDI DIRKDLYANN VMSGGTTMYP GIADRMQKEI TAL APSTMK IKIIAPPERK YSVWIGGSIL ASLSTFQQMW ITKQEYDEAG PSIVHRKCF UniProtKB: Actin, alpha skeletal muscle |
-Macromolecule #4: Deoxyribonuclease-1
| Macromolecule | Name: Deoxyribonuclease-1 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO / EC number: deoxyribonuclease I |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 29.092574 KDa |
| Sequence | String: LKIAAFNIRT FGETKMSNAT LASYIVRIVR RYDIVLIQEV RDSHLVAVGK LLDYLNQDDP NTYHYVVSEP LGRNSYKERY LFLFRPNKV SVLDTYQYDD GCESCGNDSF SREPAVVKFS SHSTKVKEFA IVALHSAPSD AVAEINSLYD VYLDVQQKWH L NDVMLMGD ...String: LKIAAFNIRT FGETKMSNAT LASYIVRIVR RYDIVLIQEV RDSHLVAVGK LLDYLNQDDP NTYHYVVSEP LGRNSYKERY LFLFRPNKV SVLDTYQYDD GCESCGNDSF SREPAVVKFS SHSTKVKEFA IVALHSAPSD AVAEINSLYD VYLDVQQKWH L NDVMLMGD FNADCSYVTS SQWSSIRLRT SSTFQWLIPD SADTTATSTN CAYDRIVVAG SLLQSSVVPG SAAPFDFQAA YG LSNEMAL AISDHYPVEV TLT UniProtKB: Deoxyribonuclease-1 |
-Macromolecule #5: Protein phosphatase 1 regulatory subunit 15A,Maltose/maltodextrin...
| Macromolecule | Name: Protein phosphatase 1 regulatory subunit 15A,Maltose/maltodextrin-binding periplasmic protein type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 49.169797 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: ARKVRFSEKV TVHFLAVWAG PAQAARQGPW EQLARDRSRF ARRITQAQEE LSPCLTPAAR ARAWARLRNP PLQAKIEEGK LVIWINGDK GYNGLAEVGK KFEKDTGIKV TVEHPDKLEE KFPQVAATGD GPDIIFWAHD RFGGYAQSGL LAEITPDKAF Q DKLYPFTW ...String: ARKVRFSEKV TVHFLAVWAG PAQAARQGPW EQLARDRSRF ARRITQAQEE LSPCLTPAAR ARAWARLRNP PLQAKIEEGK LVIWINGDK GYNGLAEVGK KFEKDTGIKV TVEHPDKLEE KFPQVAATGD GPDIIFWAHD RFGGYAQSGL LAEITPDKAF Q DKLYPFTW DAVRYNGKLI AYPIAVEALS LIYNKDLLPN PPKTWEEIPA LDKELKAKGK SALMFNLQEP YFTWPLIAAD GG YAFKYEN GKYDIKDVGV DNAGAKAGLT FLVDLIKNKH MNADTDYSIA EAAFNKGETA MTINGPWAWS NIDTSKVNYG VTV LPTFKG QPSKPFVGVL SAGINAASPN KELAKEFLEN YLLTDEGLEA VNKDKPLGAV ALKSYEEELA KDPRIAATME NAQK GEIMP NIPQMSAFWY AVRTAVINAA SGRQTVDEAL KDAQTRITK UniProtKB: Protein phosphatase 1 regulatory subunit 15A, Maltose/maltodextrin-binding periplasmic protein |
-Macromolecule #7: MANGANESE (II) ION
| Macromolecule | Name: MANGANESE (II) ION / type: ligand / ID: 7 / Number of copies: 1 / Formula: MN |
|---|---|
| Molecular weight | Theoretical: 54.938 Da |
-Macromolecule #8: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 8 / Number of copies: 1 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5 mg/mL | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: 0.22mM Triton X-100 was added into the solution before plunging. | ||||||||||||||||
| Grid | Model: UltrAuFoil R0.6/1 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 180 sec. / Pretreatment - Atmosphere: OTHER / Details: current 25mA at Pelco EasiGLOW | ||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Phase plate: VOLTA PHASE PLATE / Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 4025 / Average electron dose: 46.84 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: -2.8000000000000003 µm / Nominal defocus min: -1.0 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: OTHER / Overall B value: 47 | ||||||||||
| Output model |  PDB-7nzm: |
 Movie
Movie Controller
Controller


























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)