[English] 日本語
 Yorodumi
Yorodumi- EMDB-10095: Structure of the FliPQR complex from the flagellar type 3 secreti... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10095 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the FliPQR complex from the flagellar type 3 secretion system of Pseudomonas savastanoi. | ||||||||||||||||||
 Map data Map data | Structure of the FliPQR complex from the flagellar type 3 secretion system of Pseudomonas savastanoi. | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | flagella / T3SS / export apparatus / export gate / PROTEIN TRANSPORT | ||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationbacterial-type flagellum organization / bacterial-type flagellum basal body / bacterial-type flagellum assembly / protein secretion / protein targeting / plasma membrane Similarity search - Function | ||||||||||||||||||
| Biological species |  Pseudomonas savastanoi pv. phaseolicola 1448A (bacteria) / Pseudomonas savastanoi pv. phaseolicola 1448A (bacteria) /  Pseudomonas savastanoi pv. phaseolicola (strain 1448A / Race 6) (bacteria) / Pseudomonas savastanoi pv. phaseolicola (strain 1448A / Race 6) (bacteria) /  Pseudomonas savastanoi pv. phaseolicola (bacteria) Pseudomonas savastanoi pv. phaseolicola (bacteria) | ||||||||||||||||||
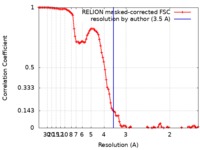
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | ||||||||||||||||||
 Authors Authors | Kuhlen L / Johnson S | ||||||||||||||||||
| Funding support |  United Kingdom, 5 items United Kingdom, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2020 Journal: Nat Commun / Year: 2020Title: The substrate specificity switch FlhB assembles onto the export gate to regulate type three secretion. Authors: Lucas Kuhlen / Steven Johnson / Andreas Zeitler / Sandra Bäurle / Justin C Deme / Joseph J E Caesar / Rebecca Debo / Joseph Fisher / Samuel Wagner / Susan M Lea /   Abstract: Protein secretion through type-three secretion systems (T3SS) is critical for motility and virulence of many bacteria. Proteins are transported through an export gate containing three proteins ...Protein secretion through type-three secretion systems (T3SS) is critical for motility and virulence of many bacteria. Proteins are transported through an export gate containing three proteins (FliPQR in flagella, SctRST in virulence systems). A fourth essential T3SS protein (FlhB/SctU) functions to "switch" secretion substrate specificity once the growing hook/needle reach their determined length. Here, we present the cryo-electron microscopy structure of an export gate containing the switch protein from a Vibrio flagellar system at 3.2 Å resolution. The structure reveals that FlhB/SctU extends the helical export gate with its four predicted transmembrane helices wrapped around FliPQR/SctRST. The unusual topology of the FlhB/SctU helices creates a loop wrapped around the bottom of the closed export gate. Structure-informed mutagenesis suggests that this loop is critical in gating secretion and we propose that a series of conformational changes in the T3SS trigger opening of the gate through interactions between FlhB/SctU and FliPQR/SctRST. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10095.map.gz emd_10095.map.gz | 85.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10095-v30.xml emd-10095-v30.xml emd-10095.xml emd-10095.xml | 22.9 KB 22.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10095_fsc.xml emd_10095_fsc.xml | 10.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_10095.png emd_10095.png | 27.9 KB | ||
| Masks |  emd_10095_msk_1.map emd_10095_msk_1.map | 91.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-10095.cif.gz emd-10095.cif.gz | 6.3 KB | ||
| Others |  emd_10095_additional.map.gz emd_10095_additional.map.gz emd_10095_half_map_1.map.gz emd_10095_half_map_1.map.gz emd_10095_half_map_2.map.gz emd_10095_half_map_2.map.gz | 71 MB 71.3 MB 71.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10095 http://ftp.pdbj.org/pub/emdb/structures/EMD-10095 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10095 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10095 | HTTPS FTP |
-Validation report
| Summary document |  emd_10095_validation.pdf.gz emd_10095_validation.pdf.gz | 1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_10095_full_validation.pdf.gz emd_10095_full_validation.pdf.gz | 1 MB | Display | |
| Data in XML |  emd_10095_validation.xml.gz emd_10095_validation.xml.gz | 17.4 KB | Display | |
| Data in CIF |  emd_10095_validation.cif.gz emd_10095_validation.cif.gz | 23 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10095 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10095 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10095 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10095 | HTTPS FTP |
-Related structure data
| Related structure data |  6s3rMC  6s3lC  6s3sC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_10095.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10095.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Structure of the FliPQR complex from the flagellar type 3 secretion system of Pseudomonas savastanoi. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.822 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
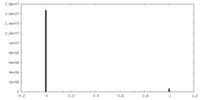
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_10095_msk_1.map emd_10095_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
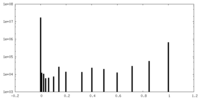
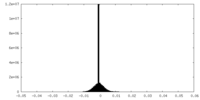
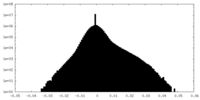
| Density Histograms |
-Additional map: refinement map
| File | emd_10095_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | refinement map | ||||||||||||
| Projections & Slices |
| ||||||||||||
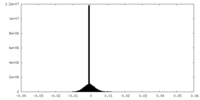
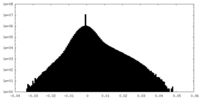
| Density Histograms |
-Half map: half map
| File | emd_10095_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
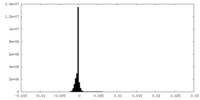
| Density Histograms |
-Half map: half map
| File | emd_10095_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
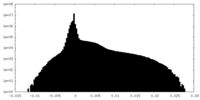
| Density Histograms |
- Sample components
Sample components
-Entire : FliPQR complex from the flagellar type 3 secretion system of Pseu...
| Entire | Name: FliPQR complex from the flagellar type 3 secretion system of Pseudomonas savastanoi |
|---|---|
| Components |
|
-Supramolecule #1: FliPQR complex from the flagellar type 3 secretion system of Pseu...
| Supramolecule | Name: FliPQR complex from the flagellar type 3 secretion system of Pseudomonas savastanoi type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Pseudomonas savastanoi pv. phaseolicola 1448A (bacteria) Pseudomonas savastanoi pv. phaseolicola 1448A (bacteria) |
| Molecular weight | Theoretical: 210 KDa |
-Macromolecule #1: Flagellar biosynthetic protein FliP
| Macromolecule | Name: Flagellar biosynthetic protein FliP / type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudomonas savastanoi pv. phaseolicola (strain 1448A / Race 6) (bacteria) Pseudomonas savastanoi pv. phaseolicola (strain 1448A / Race 6) (bacteria)Strain: 1448A / Race 6 |
| Molecular weight | Theoretical: 27.199805 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGALRFVILL LLVMVTPAVL AADPLSIPAI TLSNGADGQQ EYSVSLQILL IMTALSFIPA FVMLMTSFTR IIIVFSILRQ ALGLQQTPS NQILTGMALF LTMFIMAPVF DRVNQDALQP YLAEKLSAQD AVAKAQVPIK DFMLAQTRTS DLELFMRLSK R TDIPTPDA ...String: MGALRFVILL LLVMVTPAVL AADPLSIPAI TLSNGADGQQ EYSVSLQILL IMTALSFIPA FVMLMTSFTR IIIVFSILRQ ALGLQQTPS NQILTGMALF LTMFIMAPVF DRVNQDALQP YLAEKLSAQD AVAKAQVPIK DFMLAQTRTS DLELFMRLSK R TDIPTPDA APLTILVPAF VISELKTAFQ IGFMIFIPFL IIDLVVASVL MAMGMMMLSP LIISLPFKIM LFVLVDGWAL IV GTLAGSF GGV UniProtKB: Flagellar biosynthetic protein FliP |
-Macromolecule #2: Flagellar biosynthetic protein FliR
| Macromolecule | Name: Flagellar biosynthetic protein FliR / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudomonas savastanoi pv. phaseolicola (bacteria) Pseudomonas savastanoi pv. phaseolicola (bacteria) |
| Molecular weight | Theoretical: 32.352314 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MQPMLALTDI QISTWVASFM LPMFRIVALL MTMPVIGTTL VPRRVRLYLA FAITVVVAPA LPAMPPVQAL DLSGLLLIGE QIIIGAGMG LSLQMFFHIF VIAGQIISTQ MGMGFASMVD PTNGVSSAVI GQFFTMLVTL LFLFMNGHLV VLEVLVESFT T MPVGGGLL ...String: MQPMLALTDI QISTWVASFM LPMFRIVALL MTMPVIGTTL VPRRVRLYLA FAITVVVAPA LPAMPPVQAL DLSGLLLIGE QIIIGAGMG LSLQMFFHIF VIAGQIISTQ MGMGFASMVD PTNGVSSAVI GQFFTMLVTL LFLFMNGHLV VLEVLVESFT T MPVGGGLL VNNFWELANG LGWALSSGLR LVLPAITALL IINIAFGVMT RAAPQLNIFS IGFPLTLVLG MVILWMSMGD IL NQYQPIA SQALQSLRDM VRARENLYFQ GQFGSWSHPQ FEKGGGSGGG SGGGSWSHPQ FEK UniProtKB: Flagellar biosynthetic protein FliR |
-Macromolecule #3: Flagellar biosynthetic protein FliQ
| Macromolecule | Name: Flagellar biosynthetic protein FliQ / type: protein_or_peptide / ID: 3 / Number of copies: 5 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudomonas savastanoi pv. phaseolicola (strain 1448A / Race 6) (bacteria) Pseudomonas savastanoi pv. phaseolicola (strain 1448A / Race 6) (bacteria)Strain: 1448A / Race 6 |
| Molecular weight | Theoretical: 9.925104 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MTPEVAVDLF REALWLTTVL VAILVVPSLL CGLLVAMFQA ATQINEQTLS FLPRLLVMLV TLIVIGPWLL KIFMEYMLSL YTSIPTLIG UniProtKB: Flagellar biosynthetic protein FliQ |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4.1 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| |||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 295 K / Instrument: FEI VITROBOT MARK IV / Details: 10 seconds wait time before blotting. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 48.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 0.5 µm / Nominal magnification: 165000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-6s3r: |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)