[English] 日本語
 Yorodumi
Yorodumi- EMDB-12901: Cryo-EM structure of pyrococcus furiosus apoferritin in nanofluid... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12901 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of pyrococcus furiosus apoferritin in nanofluidic channels | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Iron Storage / METAL TRANSPORT | |||||||||
| Function / homology |  Function and homology information Function and homology informationferroxidase activity / ferric iron binding / iron ion transport / ferrous iron binding / intracellular iron ion homeostasis / identical protein binding / cytosol Similarity search - Function | |||||||||
| Biological species |   Pyrococcus furiosus COM1 (archaea) Pyrococcus furiosus COM1 (archaea) | |||||||||
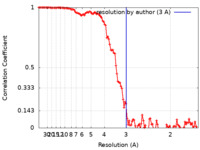
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Huber ST / Sarajlic E / Huijink R / Evers WH / Jakobi AJ | |||||||||
| Funding support | European Union,  Netherlands, 2 items Netherlands, 2 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2022 Journal: Elife / Year: 2022Title: Nanofluidic chips for cryo-EM structure determination from picoliter sample volumes. Authors: Stefan T Huber / Edin Sarajlic / Roeland Huijink / Felix Weis / Wiel H Evers / Arjen J Jakobi /   Abstract: Cryogenic electron microscopy has become an essential tool for structure determination of biological macromolecules. In practice, the difficulty to reliably prepare samples with uniform ice thickness ...Cryogenic electron microscopy has become an essential tool for structure determination of biological macromolecules. In practice, the difficulty to reliably prepare samples with uniform ice thickness still represents a barrier for routine high-resolution imaging and limits the current throughput of the technique. We show that a nanofluidic sample support with well-defined geometry can be used to prepare cryo-EM specimens with reproducible ice thickness from picoliter sample volumes. The sample solution is contained in electron-transparent nanochannels that provide uniform thickness gradients without further optimisation and eliminate the potentially destructive air-water interface. We demonstrate the possibility to perform high-resolution structure determination with three standard protein specimens. Nanofabricated sample supports bear potential to automate the cryo-EM workflow, and to explore new frontiers for cryo-EM applications such as time-resolved imaging and high-throughput screening. #1:  Journal: Biorxiv / Year: 2021 Journal: Biorxiv / Year: 2021Title: Nanofluidic chips for cryo-EM structure determination from picoliter sample volumes Authors: Huber ST / Sarajlic E / Huijink R / Weis F / Evers WH / Jakobi AJ | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12901.map.gz emd_12901.map.gz | 118 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12901-v30.xml emd-12901-v30.xml emd-12901.xml emd-12901.xml | 21.4 KB 21.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12901_fsc.xml emd_12901_fsc.xml | 11.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_12901.png emd_12901.png | 197.8 KB | ||
| Masks |  emd_12901_msk_1.map emd_12901_msk_1.map | 125 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-12901.cif.gz emd-12901.cif.gz | 5.9 KB | ||
| Others |  emd_12901_additional_1.map.gz emd_12901_additional_1.map.gz emd_12901_additional_2.map.gz emd_12901_additional_2.map.gz emd_12901_half_map_1.map.gz emd_12901_half_map_1.map.gz emd_12901_half_map_2.map.gz emd_12901_half_map_2.map.gz | 62.4 MB 9.9 MB 116 MB 116 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12901 http://ftp.pdbj.org/pub/emdb/structures/EMD-12901 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12901 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12901 | HTTPS FTP |
-Related structure data
| Related structure data |  7ohfMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10708 (Title: Apoferritin, TMV and T20S proteasome in nanofluidic channels EMPIAR-10708 (Title: Apoferritin, TMV and T20S proteasome in nanofluidic channelsData size: 308.3 Data #1: Unaligned multi-frame movies of pyrococcus furiosus apoferritin in silicon nitride nanochannels [micrographs - multiframe] Data #2: Multi-frame movies of T20S proteasome in silicon nitride nanochannels [micrographs - multiframe] Data #3: Multi-frame movies of TMV in silicon nitride nanochannels [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12901.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12901.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.8127 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_12901_msk_1.map emd_12901_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: #1
| File | emd_12901_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: #2
| File | emd_12901_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_12901_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_12901_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : 24-mer of pyrococcus furiosus apoferritin
| Entire | Name: 24-mer of pyrococcus furiosus apoferritin |
|---|---|
| Components |
|
-Supramolecule #1: 24-mer of pyrococcus furiosus apoferritin
| Supramolecule | Name: 24-mer of pyrococcus furiosus apoferritin / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Pyrococcus furiosus COM1 (archaea) Pyrococcus furiosus COM1 (archaea) |
| Molecular weight | Theoretical: 492 KDa |
-Macromolecule #1: Ferritin
| Macromolecule | Name: Ferritin / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Pyrococcus furiosus COM1 (archaea) Pyrococcus furiosus COM1 (archaea) |
| Molecular weight | Theoretical: 20.535395 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAMLSERMLK ALNDQLNREL YSAYLYFAMA AYFEDLGLEG FANWMKAQAE EEIGHALRFY NYIYDRNGRV ELDEIPKPPK EWESPLKAF EAAYEHEKFI SKSIYELAAL AEEEKDYSTR AFLEWFINEQ VEEEASVKKI LDKLKFAKDS PQILFMLDKE L SARAPKLP GLLMQGGE UniProtKB: Ferritin |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.4 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||
| Grid | Model: Homemade / Material: SILICON NITRIDE | |||||||||
| Vitrification | Cryogen name: ETHANE / Instrument: LEICA PLUNGER Details: The sample was filled into cryoChips through the cantilever and then transferred within ~10 seconds to the Leica plunger for freezing.. | |||||||||
| Details | The sample was filled into nanofluidic channels. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 3 / Number real images: 948 / Average exposure time: 9.0 sec. / Average electron dose: 63.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: -2.0 µm / Nominal defocus min: -1.0 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)