[English] 日本語
 Yorodumi
Yorodumi- EMDB-10241: Structure of the RagAB peptide importer in the 'closed-closed' state -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10241 | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the RagAB peptide importer in the 'closed-closed' state | ||||||||||||||||||||||||
 Map data Map data | Sharpened map filtered by local resolution in RELION | ||||||||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology information | ||||||||||||||||||||||||
| Biological species |  Porphyromonas gingivalis W83 (bacteria) / Porphyromonas gingivalis W83 (bacteria) /  Porphyromonas gingivalis (strain ATCC BAA-308 / W83) (bacteria) Porphyromonas gingivalis (strain ATCC BAA-308 / W83) (bacteria) | ||||||||||||||||||||||||
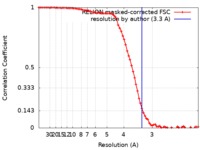
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | ||||||||||||||||||||||||
 Authors Authors | White JBR / Ranson NA / van den Berg B | ||||||||||||||||||||||||
| Funding support |  United Kingdom, United Kingdom,  Poland, 7 items Poland, 7 items
| ||||||||||||||||||||||||
 Citation Citation |  Journal: Nat Microbiol / Year: 2020 Journal: Nat Microbiol / Year: 2020Title: Structural and functional insights into oligopeptide acquisition by the RagAB transporter from Porphyromonas gingivalis. Authors: Mariusz Madej / Joshua B R White / Zuzanna Nowakowska / Shaun Rawson / Carsten Scavenius / Jan J Enghild / Grzegorz P Bereta / Karunakar Pothula / Ulrich Kleinekathoefer / Arnaud Baslé / ...Authors: Mariusz Madej / Joshua B R White / Zuzanna Nowakowska / Shaun Rawson / Carsten Scavenius / Jan J Enghild / Grzegorz P Bereta / Karunakar Pothula / Ulrich Kleinekathoefer / Arnaud Baslé / Neil A Ranson / Jan Potempa / Bert van den Berg /      Abstract: Porphyromonas gingivalis, an asaccharolytic member of the Bacteroidetes, is a keystone pathogen in human periodontitis that may also contribute to the development of other chronic inflammatory ...Porphyromonas gingivalis, an asaccharolytic member of the Bacteroidetes, is a keystone pathogen in human periodontitis that may also contribute to the development of other chronic inflammatory diseases. P. gingivalis utilizes protease-generated peptides derived from extracellular proteins for growth, but how these peptides enter the cell is not clear. Here, we identify RagAB as the outer-membrane importer for these peptides. X-ray crystal structures show that the transporter forms a dimeric RagAB complex, with the RagB substrate-binding surface-anchored lipoprotein forming a closed lid on the RagA TonB-dependent transporter. Cryo-electron microscopy structures reveal the opening of the RagB lid and thus provide direct evidence for a 'pedal bin' mechanism of nutrient uptake. Together with mutagenesis, peptide-binding studies and RagAB peptidomics, our work identifies RagAB as a dynamic, selective outer-membrane oligopeptide-acquisition machine that is essential for the efficient utilization of proteinaceous nutrients by P. gingivalis. | ||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10241.map.gz emd_10241.map.gz | 24.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10241-v30.xml emd-10241-v30.xml emd-10241.xml emd-10241.xml | 24 KB 24 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10241_fsc.xml emd_10241_fsc.xml | 7.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_10241.png emd_10241.png | 55.8 KB | ||
| Masks |  emd_10241_msk_1.map emd_10241_msk_1.map | 38.4 MB |  Mask map Mask map | |
| Others |  emd_10241_additional_1.map.gz emd_10241_additional_1.map.gz emd_10241_additional_2.map.gz emd_10241_additional_2.map.gz emd_10241_additional_3.map.gz emd_10241_additional_3.map.gz | 28.4 MB 14.1 MB 14.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10241 http://ftp.pdbj.org/pub/emdb/structures/EMD-10241 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10241 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10241 | HTTPS FTP |
-Validation report
| Summary document |  emd_10241_validation.pdf.gz emd_10241_validation.pdf.gz | 298.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_10241_full_validation.pdf.gz emd_10241_full_validation.pdf.gz | 297.9 KB | Display | |
| Data in XML |  emd_10241_validation.xml.gz emd_10241_validation.xml.gz | 10 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10241 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10241 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10241 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10241 | HTTPS FTP |
-Related structure data
| Related structure data |  6sm3MC  6sliC  6sljC  6slnC  6smlC  6smqC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10543 (Title: Cryo electron microscopy of RagAB from P. gingivalis solubilised in DDM EMPIAR-10543 (Title: Cryo electron microscopy of RagAB from P. gingivalis solubilised in DDMData size: 9.0 TB Data #1: Raw micrograph movies of the RagAB complex solubilised in DDM [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_10241.map.gz / Format: CCP4 / Size: 38.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10241.map.gz / Format: CCP4 / Size: 38.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened map filtered by local resolution in RELION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_10241_msk_1.map emd_10241_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Unsharpened map from 3D auto-refinement in RELION
| File | emd_10241_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Unsharpened map from 3D auto-refinement in RELION | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Half map 2 from the final iteration of 3D auto-refinement in RELION
| File | emd_10241_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 from the final iteration of 3D auto-refinement in RELION | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Half map 1 from the final iteration of 3D auto-refinement in RELION
| File | emd_10241_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 from the final iteration of 3D auto-refinement in RELION | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : RagAB with putative peptide substrate
| Entire | Name: RagAB with putative peptide substrate |
|---|---|
| Components |
|
-Supramolecule #1: RagAB with putative peptide substrate
| Supramolecule | Name: RagAB with putative peptide substrate / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 Details: Putative peptide substrate co-purified with the complex |
|---|---|
| Source (natural) | Organism:  Porphyromonas gingivalis W83 (bacteria) / Strain: KRAB Porphyromonas gingivalis W83 (bacteria) / Strain: KRAB |
-Macromolecule #1: Lipoprotein RagB
| Macromolecule | Name: Lipoprotein RagB / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Porphyromonas gingivalis (strain ATCC BAA-308 / W83) (bacteria) Porphyromonas gingivalis (strain ATCC BAA-308 / W83) (bacteria)Strain: ATCC BAA-308 / W83 |
| Molecular weight | Theoretical: 54.430863 KDa |
| Sequence | String: CELDRDPEGK DFQQPYTSFV QTKQNRDGLY ALLRNTENPR MHFYQELQSD MYCTTITDGN SLAPFVNWDL GILNDHGRAD EDEVSGIAG YYFVYNRLNQ QANAFVNNTE AALQNQVYKN STEIANAKSF LAEGKVLQAL AIWRLMDRFS FHESVTEVNS G AKDLGVIL ...String: CELDRDPEGK DFQQPYTSFV QTKQNRDGLY ALLRNTENPR MHFYQELQSD MYCTTITDGN SLAPFVNWDL GILNDHGRAD EDEVSGIAG YYFVYNRLNQ QANAFVNNTE AALQNQVYKN STEIANAKSF LAEGKVLQAL AIWRLMDRFS FHESVTEVNS G AKDLGVIL LKEYNPGYIG PRATKAQCYD YILSRLSEAI EVLPENRESV LYVSRDYAYA LRARIYLALG EYGKAAADAK MV VDKYPLI GAADASEFEN IYRSDANNPE IIFRGFASAT LGSFTATTLN GAAPAGKDIK YNPSAVPFQW VVDLYENEDF RKS VYIAKV VKKDKGYLVN KFLEDKAYRD VQDKPNLKVG ARYFSVAEVY LILVESALQT GDTPTAEKYL KALSKARGAE VSVV NMEAL QAERTRELIG EGSRLRDMVR WSIPNNHDAF ETQPGLEGFA NTTPLKAQAP VGFYAYTWEF PQRDRQTNPQ LIKNW PI |
-Macromolecule #2: RagA protein
| Macromolecule | Name: RagA protein / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Porphyromonas gingivalis (strain ATCC BAA-308 / W83) (bacteria) Porphyromonas gingivalis (strain ATCC BAA-308 / W83) (bacteria)Strain: ATCC BAA-308 / W83 |
| Molecular weight | Theoretical: 100.350977 KDa |
| Sequence | String: LSTVSGSVAK VSSEKLAEKP VANIMDALQG QVAGMQVMTT SGDPTAVASV EIHGTGSLGA SSAPLYIVDG MQTSLDVVAT MNPNDFESM SVLKDASATS IYGARAANGV VFIQTKKGKM SERGRITFNA SYGISQILNT KPLDNMMTGD ELLDFQVKAG F WGNNQTVQ ...String: LSTVSGSVAK VSSEKLAEKP VANIMDALQG QVAGMQVMTT SGDPTAVASV EIHGTGSLGA SSAPLYIVDG MQTSLDVVAT MNPNDFESM SVLKDASATS IYGARAANGV VFIQTKKGKM SERGRITFNA SYGISQILNT KPLDNMMTGD ELLDFQVKAG F WGNNQTVQ KVKDMILAGA EDLYGNYDSL KDEYGKTLFP VDFNHDADWL KALFKTAPTS QGDISFSGGS QGTSYYASIG YF DQEGMAR EPANFKRYSG RLNFESRINE WLKVGANLSG AIANRRSADY FGKYYMGSGT FGVLTMPRYY NPFDVNGDLA DVY YMYGAT RPSMTEPYFA KMRPFSSESH QANVNGFAQI TPIKGLTLKA QAGVDITNTR TSSKRMPNNP YDSTPLGERR ERAY RDVSK SFTNTAEYKF SIDEKHDLTA LMGHEYIEYE GDVIGASSKG FESDKLMLLS QGKTGNSLSL PEHRVAEYAY LSFFS RFNY GFDKWMYIDF SVRNDQSSRF GSNNRSAWFY SVGGMFDIYN KFIQESNWLS DLRLKMSYGT TGNSEIGNYN HQALVT VNN YTEDAMGLSI STAGNPDLSW EKQSQFNFGL AAGAFNNRLS AEVDFYVRTT NDMLIDVPMP YISGFFSQYQ NVGSMKN TG VDLSLKGTIY QNKDWNVYAS ANFNYNRQEI TKLFFGLNKY MLPNTGTIWE IGYPNSFYMA EYAGIDKKTG KQLWYVPG Q VDADGNKVTT SQYSADLETR IDKSVTPPIT GGFSLGASWK GLSLDADFAY IVGKWMINND RYFTENGGGL MQLNKDKML LNAWTEDNKE TDVPKLGQSP QFDTHLLENA SFLRLKNLKL TYVLPNSLFA GQNVIGGARV YLMARNLLTV TKYKGFDPEA GGNVGKNQY PNSKQYVAGI QLSF |
-Macromolecule #3: GLY-GLY-ALA-THR-THR-ALA-THR-THR-THR-THR-SER-THR-SER
| Macromolecule | Name: GLY-GLY-ALA-THR-THR-ALA-THR-THR-THR-THR-SER-THR-SER / type: protein_or_peptide / ID: 3 / Details: Putative peptide substrate / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Porphyromonas gingivalis W83 (bacteria) / Strain: KRAB Porphyromonas gingivalis W83 (bacteria) / Strain: KRAB |
| Molecular weight | Theoretical: 1.156157 KDa |
| Sequence | String: GGATTATTTT STS |
-Macromolecule #4: (1R,4S,6R)-6-({[2-(ACETYLAMINO)-2-DEOXY-ALPHA-D-GLUCOPYRANOSYL]OX...
| Macromolecule | Name: (1R,4S,6R)-6-({[2-(ACETYLAMINO)-2-DEOXY-ALPHA-D-GLUCOPYRANOSYL]OXY}METHYL)-4-HYDROXY-1-{[(15-METHYLHEXADECANOYL)OXY]METHYL}-4-OXIDO-7-OXO-3,5-DIOXA-8-AZA-4-PHOSPHAHEPTACOS-1-YL 15-METHYLHEXADECANOATE type: ligand / ID: 4 / Number of copies: 1 / Formula: 5PL |
|---|---|
| Molecular weight | Theoretical: 1.233719 KDa |
| Chemical component information |  ChemComp-5PL: |
-Macromolecule #5: PALMITIC ACID
| Macromolecule | Name: PALMITIC ACID / type: ligand / ID: 5 / Number of copies: 1 / Formula: PLM |
|---|---|
| Molecular weight | Theoretical: 256.424 Da |
| Chemical component information |  ChemComp-PLM: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.75 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Pretreatment - Type: GLOW DISCHARGE | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 279.15 K / Instrument: FEI VITROBOT MARK IV / Details: 6 second blot time, blot force 6. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 77.88 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)