+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0919 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the killifish CALHM1 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | channel / MEMBRANE PROTEIN | |||||||||
| Function / homology | Calcium homeostasis modulator family / Calcium homeostasis modulator / ATP export / monoatomic cation channel activity / calcium channel activity / basolateral plasma membrane / endoplasmic reticulum membrane / plasma membrane / Calcium homeostasis modulator 1 Function and homology information Function and homology information | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.66 Å | |||||||||
 Authors Authors | Demura K / Kusakizako T | |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2020 Journal: Sci Adv / Year: 2020Title: Cryo-EM structures of calcium homeostasis modulator channels in diverse oligomeric assemblies. Authors: Kanae Demura / Tsukasa Kusakizako / Wataru Shihoya / Masahiro Hiraizumi / Kengo Nomura / Hiroto Shimada / Keitaro Yamashita / Tomohiro Nishizawa / Akiyuki Taruno / Osamu Nureki /  Abstract: Calcium homeostasis modulator (CALHM) family proteins are Ca-regulated adenosine triphosphate (ATP)-release channels involved in neural functions including neurotransmission in gustation. Here, we ...Calcium homeostasis modulator (CALHM) family proteins are Ca-regulated adenosine triphosphate (ATP)-release channels involved in neural functions including neurotransmission in gustation. Here, we present the cryo-electron microscopy (EM) structures of killifish CALHM1, human CALHM2, and CLHM-1 at resolutions of 2.66, 3.4, and 3.6 Å, respectively. The CALHM1 octamer structure reveals that the N-terminal helix forms the constriction site at the channel pore in the open state and modulates the ATP conductance. The CALHM2 undecamer and CLHM-1 nonamer structures show the different oligomeric stoichiometries among CALHM homologs. We further report the cryo-EM structures of the chimeric construct, revealing that the intersubunit interactions at the transmembrane domain (TMD) and the TMD-intracellular domain linker define the oligomeric stoichiometry. These findings advance our understanding of the ATP conduction and oligomerization mechanisms of CALHM channels. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0919.map.gz emd_0919.map.gz | 135.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0919-v30.xml emd-0919-v30.xml emd-0919.xml emd-0919.xml | 17.1 KB 17.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_0919_fsc.xml emd_0919_fsc.xml | 13 KB | Display |  FSC data file FSC data file |
| Images |  emd_0919.png emd_0919.png | 142.6 KB | ||
| Masks |  emd_0919_msk_1.map emd_0919_msk_1.map | 190.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-0919.cif.gz emd-0919.cif.gz | 5.9 KB | ||
| Others |  emd_0919_half_map_1.map.gz emd_0919_half_map_1.map.gz emd_0919_half_map_2.map.gz emd_0919_half_map_2.map.gz | 145.3 MB 145.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0919 http://ftp.pdbj.org/pub/emdb/structures/EMD-0919 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0919 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0919 | HTTPS FTP |
-Validation report
| Summary document |  emd_0919_validation.pdf.gz emd_0919_validation.pdf.gz | 805.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_0919_full_validation.pdf.gz emd_0919_full_validation.pdf.gz | 804.8 KB | Display | |
| Data in XML |  emd_0919_validation.xml.gz emd_0919_validation.xml.gz | 20.6 KB | Display | |
| Data in CIF |  emd_0919_validation.cif.gz emd_0919_validation.cif.gz | 27.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0919 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0919 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0919 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0919 | HTTPS FTP |
-Related structure data
| Related structure data |  6lmtMC  0920C  0921C  0922C  0923C  6lmuC  6lmvC  6lmwC  6lmxC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10444 (Title: Cryo-EM structures of calcium homeostasis modulator (CALHM) channels EMPIAR-10444 (Title: Cryo-EM structures of calcium homeostasis modulator (CALHM) channelsData size: 6.8 TB Data #1: Unaligned movies for OlCALHM1 [micrographs - multiframe] Data #2: Unaligned movies for HsCALHM2 [micrographs - multiframe] Data #3: Unaligned movies for CeCLHM-1 [micrographs - multiframe] Data #4: Unaligned movies for OlCALHM1-HsCALHM2 chimera [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_0919.map.gz / Format: CCP4 / Size: 190.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0919.map.gz / Format: CCP4 / Size: 190.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
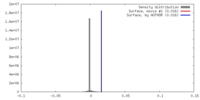
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_0919_msk_1.map emd_0919_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
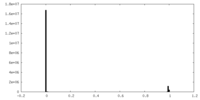
| Projections & Slices |
| ||||||||||||
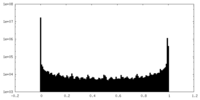
| Density Histograms |
-Half map: half map 1
| File | emd_0919_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
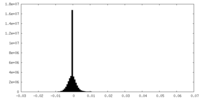
| Density Histograms |
-Half map: half map 2
| File | emd_0919_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : CALHM1
| Entire | Name: CALHM1 |
|---|---|
| Components |
|
-Supramolecule #1: CALHM1
| Supramolecule | Name: CALHM1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Calcium homeostasis modulator 1
| Macromolecule | Name: Calcium homeostasis modulator 1 / type: protein_or_peptide / ID: 1 / Number of copies: 8 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 35.039684 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDKFRMMFQF LQSNQESFMN GICGIMALAS AQMYSSFEFS CPCMPEYNYT YGIGLLIIPP IWFFLLGFVL NNNVSVLAEE WKRPTGRRT KDPSVLRYML CSITQRSLIA PAVWVSVTLM DGKSFLCAFS INLDIEKFGN ASLVIGMTET EKLKFLARIP C KDLFEDNE ...String: MDKFRMMFQF LQSNQESFMN GICGIMALAS AQMYSSFEFS CPCMPEYNYT YGIGLLIIPP IWFFLLGFVL NNNVSVLAEE WKRPTGRRT KDPSVLRYML CSITQRSLIA PAVWVSVTLM DGKSFLCAFS INLDIEKFGN ASLVIGMTET EKLKFLARIP C KDLFEDNE VRVAATRYIK CISQACGWMF LLMMTFTAFL IRAIRPCFTQ AAFLKTKYWS HYIDIERKMF DETCKEHAKS FA KVCIHQY FENISGEMQN FHRHQSKDTS DAEEEEKQRS DEDKLLGIKA QEDMNKVLEN LYFQ UniProtKB: Calcium homeostasis modulator 1 |
-Macromolecule #2: CHOLESTEROL HEMISUCCINATE
| Macromolecule | Name: CHOLESTEROL HEMISUCCINATE / type: ligand / ID: 2 / Number of copies: 32 / Formula: Y01 |
|---|---|
| Molecular weight | Theoretical: 486.726 Da |
| Chemical component information |  ChemComp-Y01: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 59.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)