+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9850 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | CD98hc extracellular domain bound to HBJ127 Fab and MEM-108 Fab | ||||||||||||
 Map data Map data | Masked, sharpend map calculated with the mask covering CD98hc-ED and two Fabs. | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Transporter / Glycoprotein / Complex / MEMBRANE PROTEIN / MEMBRANE PROTEIN-IMMUNE SYSTEM complex | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationapical pole of neuron / tyrosine transport / L-histidine transport / amino acid transport complex / L-leucine import across plasma membrane / L-alanine transmembrane transporter activity / L-alanine import across plasma membrane / Defective SLC7A7 causes lysinuric protein intolerance (LPI) / aromatic amino acid transmembrane transporter activity / phenylalanine transport ...apical pole of neuron / tyrosine transport / L-histidine transport / amino acid transport complex / L-leucine import across plasma membrane / L-alanine transmembrane transporter activity / L-alanine import across plasma membrane / Defective SLC7A7 causes lysinuric protein intolerance (LPI) / aromatic amino acid transmembrane transporter activity / phenylalanine transport / methionine transport / L-leucine transmembrane transporter activity / isoleucine transport / valine transport / proline transport / L-leucine transport / thyroid hormone transport / neutral L-amino acid transmembrane transporter activity / Tryptophan catabolism / exogenous protein binding / Amino acid transport across the plasma membrane / anchoring junction / Basigin interactions / response to exogenous dsRNA / amino acid transport / tryptophan transport / basal plasma membrane / calcium ion transport / melanosome / double-stranded RNA binding / virus receptor activity / basolateral plasma membrane / carbohydrate metabolic process / apical plasma membrane / cadherin binding / protein heterodimerization activity / lysosomal membrane / symbiont entry into host cell / synapse / cell surface / protein homodimerization activity / RNA binding / extracellular exosome / nucleoplasm / membrane / plasma membrane Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | ||||||||||||
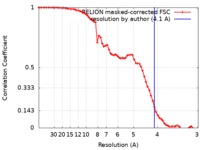
| Method | single particle reconstruction / cryo EM / Resolution: 4.1 Å | ||||||||||||
 Authors Authors | Lee Y / Nishizawa T | ||||||||||||
| Funding support |  Japan, 3 items Japan, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2019 Journal: Nat Struct Mol Biol / Year: 2019Title: Cryo-EM structure of the human L-type amino acid transporter 1 in complex with glycoprotein CD98hc. Authors: Yongchan Lee / Pattama Wiriyasermkul / Chunhuan Jin / Lili Quan / Ryuichi Ohgaki / Suguru Okuda / Tsukasa Kusakizako / Tomohiro Nishizawa / Kazumasa Oda / Ryuichiro Ishitani / Takeshi ...Authors: Yongchan Lee / Pattama Wiriyasermkul / Chunhuan Jin / Lili Quan / Ryuichi Ohgaki / Suguru Okuda / Tsukasa Kusakizako / Tomohiro Nishizawa / Kazumasa Oda / Ryuichiro Ishitani / Takeshi Yokoyama / Takanori Nakane / Mikako Shirouzu / Hitoshi Endou / Shushi Nagamori / Yoshikatsu Kanai / Osamu Nureki /    Abstract: The L-type amino acid transporter 1 (LAT1 or SLC7A5) transports large neutral amino acids across the membrane and is crucial for brain drug delivery and tumor growth. LAT1 forms a disulfide-linked ...The L-type amino acid transporter 1 (LAT1 or SLC7A5) transports large neutral amino acids across the membrane and is crucial for brain drug delivery and tumor growth. LAT1 forms a disulfide-linked heterodimer with CD98 heavy chain (CD98hc, 4F2hc or SLC3A2), but the mechanism of assembly and amino acid transport are poorly understood. Here we report the cryo-EM structure of the human LAT1-CD98hc heterodimer at 3.3-Å resolution. LAT1 features a canonical Leu T-fold and exhibits an unusual loop structure on transmembrane helix 6, creating an extended cavity that might accommodate bulky amino acids and drugs. CD98hc engages with LAT1 through the extracellular, transmembrane and putative cholesterol-mediated interactions. We also show that two anti-CD98 antibodies recognize distinct, multiple epitopes on CD98hc but not its glycans, explaining their robust reactivities. These results reveal the principles of glycoprotein-solute carrier assembly and provide templates for improving preclinical drugs and antibodies targeting LAT1 or CD98hc. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9850.map.gz emd_9850.map.gz | 2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9850-v30.xml emd-9850-v30.xml emd-9850.xml emd-9850.xml | 26.7 KB 26.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_9850_fsc.xml emd_9850_fsc.xml | 6.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_9850.png emd_9850.png | 145.5 KB | ||
| Masks |  emd_9850_msk_1.map emd_9850_msk_1.map | 26.2 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-9850.cif.gz emd-9850.cif.gz | 8 KB | ||
| Others |  emd_9850_half_map_1.map.gz emd_9850_half_map_1.map.gz emd_9850_half_map_2.map.gz emd_9850_half_map_2.map.gz | 20 MB 20 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9850 http://ftp.pdbj.org/pub/emdb/structures/EMD-9850 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9850 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9850 | HTTPS FTP |
-Related structure data
| Related structure data |  6jmrMC  9849C  6jmqC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10265 (Title: LAT1-CD98hc bound to HBJ127 Fab and MEM-108 Fab / Data size: 969.9 EMPIAR-10265 (Title: LAT1-CD98hc bound to HBJ127 Fab and MEM-108 Fab / Data size: 969.9 Data #1: Unaligned multi-frame micrographs [micrographs - multiframe]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_9850.map.gz / Format: CCP4 / Size: 26.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9850.map.gz / Format: CCP4 / Size: 26.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Masked, sharpend map calculated with the mask covering CD98hc-ED and two Fabs. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.49 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_9850_msk_1.map emd_9850_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 1 used for FSC calculation.
| File | emd_9850_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 used for FSC calculation. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 2 used for FSC calculation.
| File | emd_9850_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 used for FSC calculation. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : CD98hc extracellular domain bound to MEM-108 Fab and HBJ127 Fab
| Entire | Name: CD98hc extracellular domain bound to MEM-108 Fab and HBJ127 Fab |
|---|---|
| Components |
|
-Supramolecule #1: CD98hc extracellular domain bound to MEM-108 Fab and HBJ127 Fab
| Supramolecule | Name: CD98hc extracellular domain bound to MEM-108 Fab and HBJ127 Fab type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 225 kDa/nm |
-Macromolecule #1: Antibody
| Macromolecule | Name: Antibody / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 23.831834 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVKLLESGPG LVQPSQSLSI TCTVSGFSLT TYGIHWVRQP PGKGLEWLGV IWSNGRIDYN AAFISRLSIT KDNSKSQVFF KMNSLQDDD TAIYYCARNV YDSLTWFTYW GQGTLVTVSA AKTTPPSVYP LAPGSAAQTN SMVTLGCLVK GYFPEPVTVT W NSGSLSSG ...String: QVKLLESGPG LVQPSQSLSI TCTVSGFSLT TYGIHWVRQP PGKGLEWLGV IWSNGRIDYN AAFISRLSIT KDNSKSQVFF KMNSLQDDD TAIYYCARNV YDSLTWFTYW GQGTLVTVSA AKTTPPSVYP LAPGSAAQTN SMVTLGCLVK GYFPEPVTVT W NSGSLSSG VHTFPAVLQS DLYTLSSSVT VPSSTWPSET VTCNVAHPAS STKVDKKIVP RD |
-Macromolecule #2: Antibody
| Macromolecule | Name: Antibody / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 24.11467 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DIQMTQSPSS LAVSVGEKVT MTCKSSQSLL YSSNQKNYLA WYQQKPGQSP KLLIFWASTR ESGVPDRFTG SGSGTDFPLT ISSVKAEDL AVYFCQQYTS YPTFGGGTKL EIKRADAAPT VSIFPPSSEQ LTSGGASVVC FLNNFYPKDI NVKWKIDGSE R QNGVLNSW ...String: DIQMTQSPSS LAVSVGEKVT MTCKSSQSLL YSSNQKNYLA WYQQKPGQSP KLLIFWASTR ESGVPDRFTG SGSGTDFPLT ISSVKAEDL AVYFCQQYTS YPTFGGGTKL EIKRADAAPT VSIFPPSSEQ LTSGGASVVC FLNNFYPKDI NVKWKIDGSE R QNGVLNSW TDQDSKDSTY SMSSTLTLTK DEYERHNSYT CEATHKTSTS PIVKSFNRNE |
-Macromolecule #3: 4F2 cell-surface antigen heavy chain
| Macromolecule | Name: 4F2 cell-surface antigen heavy chain / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 68.069625 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GSELQPPEAS IAVVSIPRQL PGSHSEAGVQ GLSAGDDSEL GSHCVAQTGL ELLASGDPLP SASQNAEMIE TGSDCVTQAG LQLLASSDP PALASKNAEV TGTMSQDTEV DMKEVELNEL EPEKQPMNAA SGAAMSLAGA EKNGLVKIKV AEDEAEAAAA A KFTGLSKE ...String: GSELQPPEAS IAVVSIPRQL PGSHSEAGVQ GLSAGDDSEL GSHCVAQTGL ELLASGDPLP SASQNAEMIE TGSDCVTQAG LQLLASSDP PALASKNAEV TGTMSQDTEV DMKEVELNEL EPEKQPMNAA SGAAMSLAGA EKNGLVKIKV AEDEAEAAAA A KFTGLSKE ELLKVAGSPG WVRTRWALLL LFWLGWLGML AGAVVIIVRA PRCRELPAQK WWHTGALYRI GDLQAFQGHG AG NLAGLKG RLDYLSSLKV KGLVLGPIHK NQKDDVAQTD LLQIDPNFGS KEDFDSLLQS AKKKSIRVIL DLTPNYRGEN SWF STQVDT VATKVKDALE FWLQAGVDGF QVRDIENLKD ASSFLAEWQN ITKGFSEDRL LIAGTNSSDL QQILSLLESN KDLL LTSSY LSDSGSTGEH TKSLVTQYLN ATGNRWCSWS LSQARLLTSF LPAQLLRLYQ LMLFTLPGTP VFSYGDEIGL DAAAL PGQP MEAPVMLWDE SSFPDIPGAV SANMTVKGQS EDPGSLLSLF RRLSDQRSKE RSLLHGDFHA FSAGPGLFSY IRHWDQ NER FLVVLNFGDV GLSAGLQASD LPASASLPAK ADLLLSTQPG REEGSPLELE RLKLEPHEGL LLRFPYAA UniProtKB: Amino acid transporter heavy chain SLC3A2 |
-Macromolecule #4: Antibody
| Macromolecule | Name: Antibody / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 22.577857 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLKESGPG LVAPSQSLSI TCTVSGFPLT (UNK)(UNK)(UNK)(UNK)(UNK)WVRQP PGKGLEWLG(UNK) (UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)RLSI SKDNSK SQV FLKMNSLQTD ...String: QVQLKESGPG LVAPSQSLSI TCTVSGFPLT (UNK)(UNK)(UNK)(UNK)(UNK)WVRQP PGKGLEWLG(UNK) (UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)RLSI SKDNSK SQV FLKMNSLQTD DTARYYCAR(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)W GQGTSVT VS SAKTTPPSVY PLAPGS(UNK)(UNK)(UNK)(UNK) (UNK)SMVTLGCLV KGYFPEPVTV TWNSGSLSSG VHTFPAVL Q SDLYTLSSSV TVPSSTWPSE TVTCNVAHPA SSTKVDKKIV PRD |
-Macromolecule #5: Antibody
| Macromolecule | Name: Antibody / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 23.227152 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DIVMSQSPSS LVVSVGEKVT MSC(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)WYQQKPGQS PKLLIY(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) GVPDRF TGSGSGTDFT ...String: DIVMSQSPSS LVVSVGEKVT MSC(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)WYQQKPGQS PKLLIY(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) GVPDRF TGSGSGTDFT LTISSVKAED LAVYYC(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)FGG G TKLEIKRADA APTVSIFPPS SEQLTSGGAS VVCFLNNFYP KDINVKWKID GSERQNGVLN SWTDQDSKDS TYSMSSTLT LTKDEYERHN SYTCEATHKT STSPIVKSFN RNE |
-Macromolecule #7: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 7 / Number of copies: 3 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.2 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)