+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-9849 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | LAT1-CD98hc complex bound to MEM-108 Fab | |||||||||
 Map data Map data | Sharpened, masked map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Transporter / Glycoprotein / Complex / MEMBRANE PROTEIN / MEMBRANE PROTEIN-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationL-tryptophan transmembrane transport / positive regulation of L-leucine import across plasma membrane / L-tryptophan transmembrane transporter activity / cellular response to L-arginine / apical pole of neuron / tyrosine transport / L-histidine transport / amino acid transport complex / L-leucine import across plasma membrane / L-alanine transmembrane transporter activity ...L-tryptophan transmembrane transport / positive regulation of L-leucine import across plasma membrane / L-tryptophan transmembrane transporter activity / cellular response to L-arginine / apical pole of neuron / tyrosine transport / L-histidine transport / amino acid transport complex / L-leucine import across plasma membrane / L-alanine transmembrane transporter activity / L-alanine import across plasma membrane / Defective SLC7A7 causes lysinuric protein intolerance (LPI) / aromatic amino acid transmembrane transporter activity / phenylalanine transport / methionine transport / L-leucine transmembrane transporter activity / thyroid hormone transmembrane transporter activity / isoleucine transport / valine transport / amino acid transmembrane transport / proline transport / L-amino acid transmembrane transporter activity / alanine transport / L-leucine transport / thyroid hormone transport / negative regulation of vascular associated smooth muscle cell apoptotic process / neutral amino acid transport / amino acid import across plasma membrane / positive regulation of cytokine production involved in immune response / external side of apical plasma membrane / neutral L-amino acid transmembrane transporter activity / Tryptophan catabolism / exogenous protein binding / Amino acid transport across the plasma membrane / amino acid transmembrane transporter activity / anchoring junction / antiporter activity / Basigin interactions / response to muscle activity / microvillus membrane / positive regulation of interleukin-4 production / response to exogenous dsRNA / positive regulation of interleukin-17 production / amino acid transport / tryptophan transport / positive regulation of glial cell proliferation / response to hyperoxia / xenobiotic transport / transport across blood-brain barrier / cellular response to glucose starvation / liver regeneration / negative regulation of autophagy / basal plasma membrane / peptide antigen binding / positive regulation of type II interferon production / calcium ion transport / melanosome / double-stranded RNA binding / virus receptor activity / cellular response to lipopolysaccharide / basolateral plasma membrane / carbohydrate metabolic process / apical plasma membrane / cadherin binding / protein heterodimerization activity / intracellular membrane-bounded organelle / negative regulation of gene expression / lysosomal membrane / synapse / symbiont entry into host cell / cell surface / protein homodimerization activity / RNA binding / extracellular exosome / nucleoplasm / membrane / plasma membrane / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
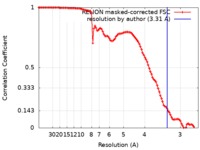
| Method | single particle reconstruction / cryo EM / Resolution: 3.31 Å | |||||||||
 Authors Authors | Lee Y / Nishizawa T | |||||||||
| Funding support |  Japan, 2 items Japan, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2019 Journal: Nat Struct Mol Biol / Year: 2019Title: Cryo-EM structure of the human L-type amino acid transporter 1 in complex with glycoprotein CD98hc. Authors: Yongchan Lee / Pattama Wiriyasermkul / Chunhuan Jin / Lili Quan / Ryuichi Ohgaki / Suguru Okuda / Tsukasa Kusakizako / Tomohiro Nishizawa / Kazumasa Oda / Ryuichiro Ishitani / Takeshi ...Authors: Yongchan Lee / Pattama Wiriyasermkul / Chunhuan Jin / Lili Quan / Ryuichi Ohgaki / Suguru Okuda / Tsukasa Kusakizako / Tomohiro Nishizawa / Kazumasa Oda / Ryuichiro Ishitani / Takeshi Yokoyama / Takanori Nakane / Mikako Shirouzu / Hitoshi Endou / Shushi Nagamori / Yoshikatsu Kanai / Osamu Nureki /    Abstract: The L-type amino acid transporter 1 (LAT1 or SLC7A5) transports large neutral amino acids across the membrane and is crucial for brain drug delivery and tumor growth. LAT1 forms a disulfide-linked ...The L-type amino acid transporter 1 (LAT1 or SLC7A5) transports large neutral amino acids across the membrane and is crucial for brain drug delivery and tumor growth. LAT1 forms a disulfide-linked heterodimer with CD98 heavy chain (CD98hc, 4F2hc or SLC3A2), but the mechanism of assembly and amino acid transport are poorly understood. Here we report the cryo-EM structure of the human LAT1-CD98hc heterodimer at 3.3-Å resolution. LAT1 features a canonical Leu T-fold and exhibits an unusual loop structure on transmembrane helix 6, creating an extended cavity that might accommodate bulky amino acids and drugs. CD98hc engages with LAT1 through the extracellular, transmembrane and putative cholesterol-mediated interactions. We also show that two anti-CD98 antibodies recognize distinct, multiple epitopes on CD98hc but not its glycans, explaining their robust reactivities. These results reveal the principles of glycoprotein-solute carrier assembly and provide templates for improving preclinical drugs and antibodies targeting LAT1 or CD98hc. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_9849.map.gz emd_9849.map.gz | 5.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-9849-v30.xml emd-9849-v30.xml emd-9849.xml emd-9849.xml | 29.2 KB 29.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_9849_fsc.xml emd_9849_fsc.xml | 10.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_9849.png emd_9849.png | 94.1 KB | ||
| Masks |  emd_9849_msk_1.map emd_9849_msk_1.map | 91.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-9849.cif.gz emd-9849.cif.gz | 8.6 KB | ||
| Others |  emd_9849_half_map_1.map.gz emd_9849_half_map_1.map.gz emd_9849_half_map_2.map.gz emd_9849_half_map_2.map.gz | 71.3 MB 71.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-9849 http://ftp.pdbj.org/pub/emdb/structures/EMD-9849 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9849 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-9849 | HTTPS FTP |
-Related structure data
| Related structure data |  6jmqMC  9850C  6jmrC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10264 (Title: LAT1-CD98hc bound to MEM-108 Fab / Data size: 4.1 TB EMPIAR-10264 (Title: LAT1-CD98hc bound to MEM-108 Fab / Data size: 4.1 TBData #1: Unaligned multi-frame micrographs, Dataset1 [micrographs - multiframe] Data #2: Unaligned multi-frame micrographs, Dataset2 [micrographs - multiframe] Data #3: Micelle subtracted particles used for final reconstruction [picked particles - single frame - processed]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_9849.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_9849.map.gz / Format: CCP4 / Size: 91.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened, masked map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.333 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_9849_msk_1.map emd_9849_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 2 used for FSC calculation. The...
| File | emd_9849_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 used for FSC calculation. The header contains a wrong pixel size information (1.375 A). The correct pixel size is 1.333 A. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 1 used for FSC calculation. The...
| File | emd_9849_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 used for FSC calculation. The header contains a wrong pixel size information (1.375 A). The correct pixel size is 1.333 A. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : LAT1-CD98hc complex bound to MEM-108 Fab
| Entire | Name: LAT1-CD98hc complex bound to MEM-108 Fab |
|---|---|
| Components |
|
-Supramolecule #1: LAT1-CD98hc complex bound to MEM-108 Fab
| Supramolecule | Name: LAT1-CD98hc complex bound to MEM-108 Fab / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 175 kDa/nm |
-Macromolecule #1: Large neutral amino acids transporter small subunit 1
| Macromolecule | Name: Large neutral amino acids transporter small subunit 1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 56.043746 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAGAGPKRRA LAAPAAEEKE EAREKMLAAK SADGSAPAGE GEGVTLQRNI TLLNGVAIIV GTIIGSGIFV TPTGVLKEAG SPGLALVVW AACGVFSIVG ALCYAELGTT ISKSGGDYAY MLEVYGSLPA FLKLWIELLI IRPSSQYIVA LVFATYLLKP L FPTCPVPE ...String: MAGAGPKRRA LAAPAAEEKE EAREKMLAAK SADGSAPAGE GEGVTLQRNI TLLNGVAIIV GTIIGSGIFV TPTGVLKEAG SPGLALVVW AACGVFSIVG ALCYAELGTT ISKSGGDYAY MLEVYGSLPA FLKLWIELLI IRPSSQYIVA LVFATYLLKP L FPTCPVPE EAAKLVACLC VLLLTAVNCY SVKAATRVQD AFAAAKLLAL ALIILLGFVQ IGKGDVSNLD PNFSFEGTKL DV GNIVLAL YSGLFAYGGW NYLNFVTEEM INPYRNLPLA IIISLPIVTL VYVLTNLAYF TTLSTEQMLS SEAVAVDFGN YHL GVMSWI IPVFVGLSCF GSVNGSLFTS SRLFFVGSRE GHLPSILSMI HPQLLTPVPS LVFTCVMTLL YAFSKDIFSV INFF SFFNW LCVALAIIGM IWLRHRKPEL ERPIKVNLAL PVFFILACLF LIAVSFWKTP VECGIGFTII LSGLPVYFFG VWWKN KPKW LLQGIFSTTV LCQKLMQVVP QETDYKDDDD K UniProtKB: Large neutral amino acids transporter small subunit 1 |
-Macromolecule #2: 4F2 cell-surface antigen heavy chain
| Macromolecule | Name: 4F2 cell-surface antigen heavy chain / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 68.069625 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GSELQPPEAS IAVVSIPRQL PGSHSEAGVQ GLSAGDDSEL GSHCVAQTGL ELLASGDPLP SASQNAEMIE TGSDCVTQAG LQLLASSDP PALASKNAEV TGTMSQDTEV DMKEVELNEL EPEKQPMNAA SGAAMSLAGA EKNGLVKIKV AEDEAEAAAA A KFTGLSKE ...String: GSELQPPEAS IAVVSIPRQL PGSHSEAGVQ GLSAGDDSEL GSHCVAQTGL ELLASGDPLP SASQNAEMIE TGSDCVTQAG LQLLASSDP PALASKNAEV TGTMSQDTEV DMKEVELNEL EPEKQPMNAA SGAAMSLAGA EKNGLVKIKV AEDEAEAAAA A KFTGLSKE ELLKVAGSPG WVRTRWALLL LFWLGWLGML AGAVVIIVRA PRCRELPAQK WWHTGALYRI GDLQAFQGHG AG NLAGLKG RLDYLSSLKV KGLVLGPIHK NQKDDVAQTD LLQIDPNFGS KEDFDSLLQS AKKKSIRVIL DLTPNYRGEN SWF STQVDT VATKVKDALE FWLQAGVDGF QVRDIENLKD ASSFLAEWQN ITKGFSEDRL LIAGTNSSDL QQILSLLESN KDLL LTSSY LSDSGSTGEH TKSLVTQYLN ATGNRWCSWS LSQARLLTSF LPAQLLRLYQ LMLFTLPGTP VFSYGDEIGL DAAAL PGQP MEAPVMLWDE SSFPDIPGAV SANMTVKGQS EDPGSLLSLF RRLSDQRSKE RSLLHGDFHA FSAGPGLFSY IRHWDQ NER FLVVLNFGDV GLSAGLQASD LPASASLPAK ADLLLSTQPG REEGSPLELE RLKLEPHEGL LLRFPYAA UniProtKB: Amino acid transporter heavy chain SLC3A2 |
-Macromolecule #3: Antibody
| Macromolecule | Name: Antibody / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 22.577857 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLKESGPG LVAPSQSLSI TCTVSGFPLT (UNK)(UNK)(UNK)(UNK)(UNK)WVRQP PGKGLEWLG(UNK) (UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)RLSI SKDNSK SQV FLKMNSLQTD ...String: QVQLKESGPG LVAPSQSLSI TCTVSGFPLT (UNK)(UNK)(UNK)(UNK)(UNK)WVRQP PGKGLEWLG(UNK) (UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)RLSI SKDNSK SQV FLKMNSLQTD DTARYYCAR(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)W GQGTSVT VS SAKTTPPSVY PLAPGS(UNK)(UNK)(UNK)(UNK) (UNK)SMVTLGCLV KGYFPEPVTV TWNSGSLSSG VHTFPAVL Q SDLYTLSSSV TVPSSTWPSE TVTCNVAHPA SSTKVDKKIV PRD |
-Macromolecule #4: Antibody
| Macromolecule | Name: Antibody / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 23.227152 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DIVMSQSPSS LVVSVGEKVT MSC(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)WYQQKPGQS PKLLIY(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) GVPDRF TGSGSGTDFT ...String: DIVMSQSPSS LVVSVGEKVT MSC(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK) (UNK)WYQQKPGQS PKLLIY(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) GVPDRF TGSGSGTDFT LTISSVKAED LAVYYC(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)FGG G TKLEIKRADA APTVSIFPPS SEQLTSGGAS VVCFLNNFYP KDINVKWKID GSERQNGVLN SWTDQDSKDS TYSMSSTLT LTKDEYERHN SYTCEATHKT STSPIVKSFN RNE |
-Macromolecule #6: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 6 / Number of copies: 5 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Macromolecule #7: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 7 / Number of copies: 3 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Average electron dose: 7.14 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)