+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-8902 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Cryo-EM Structures of ASC and NLRC4 CARD Filaments Reveal a Unified Mechanism of Nucleation and Activation of Caspase-1 | |||||||||
 マップデータ マップデータ | Helical reconstruction of ASC-CARD filament | |||||||||
 試料 試料 |
| |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報NLRP6 inflammasome complex / myosin I binding / Pyrin domain binding / myeloid dendritic cell activation involved in immune response / positive regulation of antigen processing and presentation of peptide antigen via MHC class II / negative regulation of protein serine/threonine kinase activity / regulation of intrinsic apoptotic signaling pathway / myeloid dendritic cell activation / IkappaB kinase complex / The AIM2 inflammasome ...NLRP6 inflammasome complex / myosin I binding / Pyrin domain binding / myeloid dendritic cell activation involved in immune response / positive regulation of antigen processing and presentation of peptide antigen via MHC class II / negative regulation of protein serine/threonine kinase activity / regulation of intrinsic apoptotic signaling pathway / myeloid dendritic cell activation / IkappaB kinase complex / The AIM2 inflammasome / interleukin-6 receptor binding / AIM2 inflammasome complex / icosanoid biosynthetic process / NLRP1 inflammasome complex / macropinocytosis / canonical inflammasome complex / BMP receptor binding / NLRP3 inflammasome complex assembly / NLRP3 inflammasome complex / positive regulation of adaptive immune response / cysteine-type endopeptidase activator activity / CLEC7A/inflammasome pathway / negative regulation of interferon-beta production / osmosensory signaling pathway / regulation of tumor necrosis factor-mediated signaling pathway / positive regulation of extrinsic apoptotic signaling pathway / pattern recognition receptor signaling pathway / positive regulation of macrophage cytokine production / : / pattern recognition receptor activity / tropomyosin binding / pyroptotic inflammatory response / positive regulation of actin filament polymerization / positive regulation of release of cytochrome c from mitochondria / intrinsic apoptotic signaling pathway by p53 class mediator / positive regulation of activated T cell proliferation / positive regulation of interleukin-10 production / The NLRP3 inflammasome / cellular response to interleukin-1 / intrinsic apoptotic signaling pathway in response to DNA damage by p53 class mediator / positive regulation of T cell migration / : / Purinergic signaling in leishmaniasis infection / positive regulation of chemokine production / positive regulation of defense response to virus by host / negative regulation of canonical NF-kappaB signal transduction / negative regulation of cytokine production involved in inflammatory response / intrinsic apoptotic signaling pathway / activation of innate immune response / positive regulation of phagocytosis / tumor necrosis factor-mediated signaling pathway / positive regulation of interleukin-1 beta production / positive regulation of interleukin-8 production / positive regulation of JNK cascade / apoptotic signaling pathway / positive regulation of non-canonical NF-kappaB signal transduction / : / protein homooligomerization / positive regulation of T cell activation / regulation of protein stability / positive regulation of interleukin-6 production / positive regulation of type II interferon production / positive regulation of inflammatory response / positive regulation of tumor necrosis factor production / SARS-CoV-1 activates/modulates innate immune responses / azurophil granule lumen / cellular response to tumor necrosis factor / cellular response to lipopolysaccharide / regulation of inflammatory response / protease binding / secretory granule lumen / defense response to Gram-negative bacterium / defense response to virus / microtubule / transmembrane transporter binding / positive regulation of ERK1 and ERK2 cascade / positive regulation of canonical NF-kappaB signal transduction / protein dimerization activity / defense response to Gram-positive bacterium / regulation of autophagy / positive regulation of apoptotic process / inflammatory response / Golgi membrane / innate immune response / neuronal cell body / apoptotic process / Neutrophil degranulation / nucleolus / enzyme binding / endoplasmic reticulum / signal transduction / protein homodimerization activity / protein-containing complex / mitochondrion / extracellular region / nucleoplasm / identical protein binding / nucleus / cytosol / cytoplasm 類似検索 - 分子機能 | |||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
| 手法 | らせん対称体再構成法 / クライオ電子顕微鏡法 / 解像度: 3.17 Å | |||||||||
 データ登録者 データ登録者 | Li Y / Fu T / Wu H | |||||||||
 引用 引用 |  ジャーナル: Proc Natl Acad Sci U S A / 年: 2018 ジャーナル: Proc Natl Acad Sci U S A / 年: 2018タイトル: Cryo-EM structures of ASC and NLRC4 CARD filaments reveal a unified mechanism of nucleation and activation of caspase-1. 著者: Yang Li / Tian-Min Fu / Alvin Lu / Kristen Witt / Jianbin Ruan / Chen Shen / Hao Wu /  要旨: Canonical inflammasomes are cytosolic supramolecular complexes that activate caspase-1 upon sensing extrinsic microbial invasions and intrinsic sterile stress signals. During inflammasome assembly, ...Canonical inflammasomes are cytosolic supramolecular complexes that activate caspase-1 upon sensing extrinsic microbial invasions and intrinsic sterile stress signals. During inflammasome assembly, adaptor proteins ASC and NLRC4 recruit caspase-1 through homotypic caspase recruitment domain (CARD) interactions, leading to caspase-1 dimerization and activation. Activated caspase-1 processes proinflammatory cytokines and Gasdermin D to induce cytokine maturation and pyroptotic cell death. Here, we present cryo-electron microscopy (cryo-EM) structures of NLRC4 CARD and ASC CARD filaments mediated by conserved three types of asymmetric interactions (types I, II, and III). We find that the CARDs of these two adaptor proteins share a similar assembly pattern, which matches that of the caspase-1 CARD filament whose structure we defined previously. These data indicate a unified mechanism for downstream caspase-1 recruitment through CARD-CARD interactions by both adaptors. Using structure modeling, we further show that full-length NLRC4 assembles via two separate symmetries at its CARD and its nucleotide-binding domain (NBD), respectively. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_8902.map.gz emd_8902.map.gz | 25.3 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-8902-v30.xml emd-8902-v30.xml emd-8902.xml emd-8902.xml | 9.4 KB 9.4 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_8902.png emd_8902.png | 79.1 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8902 http://ftp.pdbj.org/pub/emdb/structures/EMD-8902 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8902 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8902 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_8902.map.gz / 形式: CCP4 / 大きさ: 27 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_8902.map.gz / 形式: CCP4 / 大きさ: 27 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Helical reconstruction of ASC-CARD filament | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.32 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
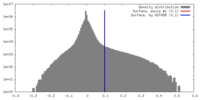
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : Caspase recruitment domain of Apoptosis-associated speck-like pro...
| 全体 | 名称: Caspase recruitment domain of Apoptosis-associated speck-like protein containing a CARD |
|---|---|
| 要素 |
|
-超分子 #1: Caspase recruitment domain of Apoptosis-associated speck-like pro...
| 超分子 | 名称: Caspase recruitment domain of Apoptosis-associated speck-like protein containing a CARD タイプ: organelle_or_cellular_component / ID: 1 / 親要素: 0 / 含まれる分子: all |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 組換発現 | 生物種: |
-分子 #1: Apoptosis-associated speck-like protein containing a CARD
| 分子 | 名称: Apoptosis-associated speck-like protein containing a CARD タイプ: protein_or_peptide / ID: 1 / コピー数: 8 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 9.775143 KDa |
| 組換発現 | 生物種: |
| 配列 | 文字列: LHFIDQHRAA LIARVTNVEW LLDALYGKVL TDEQYQAVRA EPTNPSKMRK LFSFTPAWNW TCKDLLLQAL RESQSYLVED LER |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | らせん対称体再構成法 |
| 試料の集合状態 | filament |
- 試料調製
試料調製
| 緩衝液 | pH: 8 |
|---|---|
| 凍結 | 凍結剤: ETHANE |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K2 SUMMIT (4k x 4k) 平均電子線量: 41.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
- 画像解析
画像解析
| 最終 再構成 | 想定した対称性 - らせんパラメータ - Δz: 5.0 Å 想定した対称性 - らせんパラメータ - ΔΦ: -100.58 ° 想定した対称性 - らせんパラメータ - 軸対称性: C1 (非対称) 解像度のタイプ: BY AUTHOR / 解像度: 3.17 Å / 解像度の算出法: FSC 0.143 CUT-OFF / 使用した粒子像数: 264167 |
|---|---|
| 最終 角度割当 | タイプ: NOT APPLICABLE |
 ムービー
ムービー コントローラー
コントローラー

























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)