[English] 日本語
 Yorodumi
Yorodumi- EMDB-8713: Cryo-EM reconstruction of B41 SOSIP.664 in complex with soluble C... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8713 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM reconstruction of B41 SOSIP.664 in complex with soluble CD4 (D1-D2) and fragment antigen binding of 17b | |||||||||
 Map data Map data | B41 SOSIP.664 in complex with soluble CD4 (D1-D2) and fragment antigen binding variable domain of 17b | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Viral fusion / HIV-1 / envelope glycoprotein / CD4 receptor binding / IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology informationhelper T cell enhancement of adaptive immune response / interleukin-16 binding / interleukin-16 receptor activity / maintenance of protein location in cell / T cell selection / MHC class II protein binding / positive regulation of kinase activity / cellular response to granulocyte macrophage colony-stimulating factor stimulus / interleukin-15-mediated signaling pathway / positive regulation of monocyte differentiation ...helper T cell enhancement of adaptive immune response / interleukin-16 binding / interleukin-16 receptor activity / maintenance of protein location in cell / T cell selection / MHC class II protein binding / positive regulation of kinase activity / cellular response to granulocyte macrophage colony-stimulating factor stimulus / interleukin-15-mediated signaling pathway / positive regulation of monocyte differentiation / Nef Mediated CD4 Down-regulation / Alpha-defensins / regulation of T cell activation / Other interleukin signaling / extracellular matrix structural constituent / T cell receptor complex / enzyme-linked receptor protein signaling pathway / Translocation of ZAP-70 to Immunological synapse / Phosphorylation of CD3 and TCR zeta chains / regulation of calcium ion transport / Generation of second messenger molecules / macrophage differentiation / T cell differentiation / Co-inhibition by PD-1 / positive regulation of protein kinase activity / Binding and entry of HIV virion / coreceptor activity / positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / positive regulation of interleukin-2 production / positive regulation of calcium-mediated signaling / cell surface receptor protein tyrosine kinase signaling pathway / host cell endosome membrane / protein tyrosine kinase binding / Vpu mediated degradation of CD4 / clathrin-coated endocytic vesicle membrane / calcium-mediated signaling / positive regulation of T cell activation / MHC class II protein complex binding / transmembrane signaling receptor activity / Downstream TCR signaling / Cargo recognition for clathrin-mediated endocytosis / signaling receptor activity / Clathrin-mediated endocytosis / positive regulation of protein phosphorylation / virus receptor activity / defense response to Gram-negative bacterium / clathrin-dependent endocytosis of virus by host cell / adaptive immune response / positive regulation of canonical NF-kappaB signal transduction / positive regulation of viral entry into host cell / early endosome / cell surface receptor signaling pathway / positive regulation of ERK1 and ERK2 cascade / positive regulation of MAPK cascade / cell adhesion / viral protein processing / immune response / membrane raft / endoplasmic reticulum lumen / external side of plasma membrane / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / lipid binding / viral envelope / endoplasmic reticulum membrane / protein kinase binding / positive regulation of DNA-templated transcription / virion attachment to host cell / host cell plasma membrane / virion membrane / structural molecule activity / enzyme binding / signal transduction / protein homodimerization activity / zinc ion binding / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |   Human immunodeficiency virus 1 / Human immunodeficiency virus 1 /  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Pallesen J / Ozorowski G / de Val N / Ward AB | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2017 Journal: Nature / Year: 2017Title: Open and closed structures reveal allostery and pliability in the HIV-1 envelope spike. Authors: Gabriel Ozorowski / Jesper Pallesen / Natalia de Val / Dmitry Lyumkis / Christopher A Cottrell / Jonathan L Torres / Jeffrey Copps / Robyn L Stanfield / Albert Cupo / Pavel Pugach / John P ...Authors: Gabriel Ozorowski / Jesper Pallesen / Natalia de Val / Dmitry Lyumkis / Christopher A Cottrell / Jonathan L Torres / Jeffrey Copps / Robyn L Stanfield / Albert Cupo / Pavel Pugach / John P Moore / Ian A Wilson / Andrew B Ward /  Abstract: For many enveloped viruses, binding to a receptor(s) on a host cell acts as the first step in a series of events culminating in fusion with the host cell membrane and transfer of genetic material for ...For many enveloped viruses, binding to a receptor(s) on a host cell acts as the first step in a series of events culminating in fusion with the host cell membrane and transfer of genetic material for replication. The envelope glycoprotein (Env) trimer on the surface of HIV is responsible for receptor binding and fusion. Although Env can tolerate a high degree of mutation in five variable regions (V1-V5), and also at N-linked glycosylation sites that contribute roughly half the mass of Env, the functional sites for recognition of receptor CD4 and co-receptor CXCR4/CCR5 are conserved and essential for viral fitness. Soluble SOSIP Env trimers are structural and antigenic mimics of the pre-fusion native, surface-presented Env, and are targets of broadly neutralizing antibodies. Thus, they are attractive immunogens for vaccine development. Here we present high-resolution cryo-electron microscopy structures of subtype B B41 SOSIP Env trimers in complex with CD4 and antibody 17b, or with antibody b12, at resolutions of 3.7 Å and 3.6 Å, respectively. We compare these to cryo-electron microscopy reconstructions of B41 SOSIP Env trimers with no ligand or in complex with either CD4 or the CD4-binding-site antibody PGV04 at 5.6 Å, 5.2 Å and 7.4 Å resolution, respectively. Consequently, we present the most complete description yet, to our knowledge, of the CD4-17b-induced intermediate and provide the molecular basis of the receptor-binding-induced conformational change required for HIV-1 entry into host cells. Both CD4 and b12 induce large, previously uncharacterized conformational rearrangements in the gp41 subunits, and the fusion peptide becomes buried in a newly formed pocket. These structures provide key details on the biological function of the type I viral fusion machine from HIV-1 as well as new templates for inhibitor design. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8713.map.gz emd_8713.map.gz | 60 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8713-v30.xml emd-8713-v30.xml emd-8713.xml emd-8713.xml | 30.1 KB 30.1 KB | Display Display |  EMDB header EMDB header |
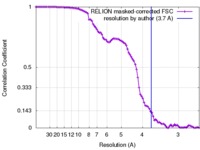
| FSC (resolution estimation) |  emd_8713_fsc.xml emd_8713_fsc.xml | 9.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_8713.png emd_8713.png | 95.6 KB | ||
| Filedesc metadata |  emd-8713.cif.gz emd-8713.cif.gz | 7.9 KB | ||
| Others |  emd_8713_additional.map.gz emd_8713_additional.map.gz emd_8713_half_map_1.map.gz emd_8713_half_map_1.map.gz emd_8713_half_map_2.map.gz emd_8713_half_map_2.map.gz | 59.9 MB 49.6 MB 49.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8713 http://ftp.pdbj.org/pub/emdb/structures/EMD-8713 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8713 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8713 | HTTPS FTP |
-Validation report
| Summary document |  emd_8713_validation.pdf.gz emd_8713_validation.pdf.gz | 882.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_8713_full_validation.pdf.gz emd_8713_full_validation.pdf.gz | 881.8 KB | Display | |
| Data in XML |  emd_8713_validation.xml.gz emd_8713_validation.xml.gz | 15.6 KB | Display | |
| Data in CIF |  emd_8713_validation.cif.gz emd_8713_validation.cif.gz | 20.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8713 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8713 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8713 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8713 | HTTPS FTP |
-Related structure data
| Related structure data |  5vn3MC  8714C  8715C  8716C  8717C  8729C  8730C  5vn8C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8713.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8713.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | B41 SOSIP.664 in complex with soluble CD4 (D1-D2) and fragment antigen binding variable domain of 17b | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.31 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: B41 SOSIP.664 in complex with soluble CD4 (D1-D2)...
| File | emd_8713_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | B41 SOSIP.664 in complex with soluble CD4 (D1-D2) and fragment antigen binding variable domain of 17b, additional map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: B41 SOSIP.664 in complex with soluble CD4 (D1-D2)...
| File | emd_8713_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | B41 SOSIP.664 in complex with soluble CD4 (D1-D2) and fragment antigen binding variable domain of 17b, half map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: B41 SOSIP.664 in complex with soluble CD4 (D1-D2)...
| File | emd_8713_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | B41 SOSIP.664 in complex with soluble CD4 (D1-D2) and fragment antigen binding variable domain of 17b, half-map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : HIV-1 Env B41 SOSIP.664 in complex with soluble CD4 (2-domain) an...
+Supramolecule #1: HIV-1 Env B41 SOSIP.664 in complex with soluble CD4 (2-domain) an...
+Supramolecule #2: HIV-1 Env B41 SOSIP.664
+Supramolecule #3: CD4 (2-domain)
+Supramolecule #4: 17b fragment antigen
+Macromolecule #1: 17b Fab light chain
+Macromolecule #2: Envelope glycoprotein gp160
+Macromolecule #3: Envelope glycoprotein gp160
+Macromolecule #4: T-cell surface glycoprotein CD4
+Macromolecule #5: 17b Fab heavy chain
+Macromolecule #11: 2-acetamido-2-deoxy-beta-D-glucopyranose
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: DDM was added to a final concentration of 0.06 mM prior to vitrification | ||||||||||||
| Grid | Model: C-flat-2/2 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 10 sec. / Pretreatment - Atmosphere: OTHER | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber temperature: 277 K / Instrument: HOMEMADE PLUNGER Details: 3 uL of sample applied to a holey carbon grid on glow discharged face and blotted manually on sample side until filter paper detached from grid, followed by immediate plunging. | ||||||||||||
| Details | B41 SOSIP.664 was incubated with a 6X molar excess of soluble CD4 and 17b Fab overnight at room temperature, purified by size exclusion chromatography, and concentrated prior to grid application |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Digitization - Frames/image: 1-50 / Number real images: 1169 / Average exposure time: 10.0 sec. / Average electron dose: 58.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated magnification: 38168 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 22500 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | Homology model of B41 SOSIP.664 created using PDB 5CEZ as initial model. sCD4 and 17b coordinates taken from PDB 1GC1. Performed fragment based refinement using Rosetta, followed by relaxed refinement in Rosetta. Modeled glycans using Chimera and performed final refinements using Phenix. |
|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Target criteria: EMRinger |
| Output model |  PDB-5vn3: |
 Movie
Movie Controller
Controller



























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)