[English] 日本語
 Yorodumi
Yorodumi- EMDB-8541: Structure of the active form of human Origin Recognition Complex ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-8541 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the active form of human Origin Recognition Complex and its ATPase motor module | |||||||||||||||
 Map data Map data | human Origin Recognition Complex and its ATPase motor module | |||||||||||||||
 Sample Sample |
| |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationpolar body extrusion after meiotic divisions / CDC6 association with the ORC:origin complex / origin recognition complex / E2F-enabled inhibition of pre-replication complex formation / nuclear origin of replication recognition complex / nuclear pre-replicative complex / inner kinetochore / DNA replication preinitiation complex / mitotic DNA replication checkpoint signaling / neural precursor cell proliferation ...polar body extrusion after meiotic divisions / CDC6 association with the ORC:origin complex / origin recognition complex / E2F-enabled inhibition of pre-replication complex formation / nuclear origin of replication recognition complex / nuclear pre-replicative complex / inner kinetochore / DNA replication preinitiation complex / mitotic DNA replication checkpoint signaling / neural precursor cell proliferation / G1/S-Specific Transcription / regulation of DNA replication / DNA replication origin binding / protein polymerization / DNA replication initiation / Activation of the pre-replicative complex / glial cell proliferation / heterochromatin / Activation of ATR in response to replication stress / Assembly of the ORC complex at the origin of replication / Assembly of the pre-replicative complex / Orc1 removal from chromatin / DNA replication / chromosome, telomeric region / nuclear body / nucleotide binding / centrosome / chromatin binding / chromatin / nucleolus / negative regulation of transcription by RNA polymerase II / ATP hydrolysis activity / DNA binding / nucleoplasm / ATP binding / membrane / nucleus / metal ion binding / cytosol Similarity search - Function | |||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 18.0 Å | |||||||||||||||
 Authors Authors | Tocilj A / On K / Yuan Z / Sun J / Elkayam E / Li H / Stillman B / Joshua-Tor L | |||||||||||||||
| Funding support |  United States, 4 items United States, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Elife / Year: 2017 Journal: Elife / Year: 2017Title: Structure of the active form of human origin recognition complex and its ATPase motor module. Authors: Ante Tocilj / Kin Fan On / Zuanning Yuan / Jingchuan Sun / Elad Elkayam / Huilin Li / Bruce Stillman / Leemor Joshua-Tor /  Abstract: Binding of the Origin Recognition Complex (ORC) to origins of replication marks the first step in the initiation of replication of the genome in all eukaryotic cells. Here, we report the structure of ...Binding of the Origin Recognition Complex (ORC) to origins of replication marks the first step in the initiation of replication of the genome in all eukaryotic cells. Here, we report the structure of the active form of human ORC determined by X-ray crystallography and cryo-electron microscopy. The complex is composed of an ORC1/4/5 motor module lobe in an organization reminiscent of the DNA polymerase clamp loader complexes. A second lobe contains the ORC2/3 subunits. The complex is organized as a double-layered shallow corkscrew, with the AAA+ and AAA+-like domains forming one layer, and the winged-helix domains (WHDs) forming a top layer. CDC6 fits easily between ORC1 and ORC2, completing the ring and the DNA-binding channel, forming an additional ATP hydrolysis site. Analysis of the ATPase activity of the complex provides a basis for understanding ORC activity as well as molecular defects observed in Meier-Gorlin Syndrome mutations. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_8541.map.gz emd_8541.map.gz | 965.6 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-8541-v30.xml emd-8541-v30.xml emd-8541.xml emd-8541.xml | 8.9 KB 8.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_8541.png emd_8541.png | 33.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-8541 http://ftp.pdbj.org/pub/emdb/structures/EMD-8541 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8541 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-8541 | HTTPS FTP |
-Validation report
| Summary document |  emd_8541_validation.pdf.gz emd_8541_validation.pdf.gz | 293.9 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_8541_full_validation.pdf.gz emd_8541_full_validation.pdf.gz | 293.4 KB | Display | |
| Data in XML |  emd_8541_validation.xml.gz emd_8541_validation.xml.gz | 4.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8541 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8541 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8541 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-8541 | HTTPS FTP |
-Related structure data
| Related structure data |  5ujmMC  8523C  5uj7C  5uj8C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_8541.map.gz / Format: CCP4 / Size: 1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_8541.map.gz / Format: CCP4 / Size: 1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | human Origin Recognition Complex and its ATPase motor module | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.04 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
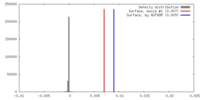
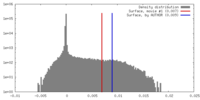
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Human ORC
| Entire | Name: Human ORC |
|---|---|
| Components |
|
-Supramolecule #1: Human ORC
| Supramolecule | Name: Human ORC / type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism: Insect cell expression vector pTIE1 (others) / Recombinant plasmid: pSPL, pFL |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 18.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 10980 |
|---|---|
| Initial angle assignment | Type: PROJECTION MATCHING |
| Final angle assignment | Type: PROJECTION MATCHING |
 Movie
Movie Controller
Controller






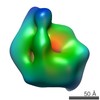









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)