[English] 日本語
 Yorodumi
Yorodumi- PDB-6t9n: CryoEM structure of human polycystin-2/PKD2 in UDM supplemented w... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6t9n | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of human polycystin-2/PKD2 in UDM supplemented with PI(4,5)P2 | |||||||||
 Components Components | Polycystin-2 | |||||||||
 Keywords Keywords | MEMBRANE PROTEIN / ION CHANNEL / TRANSIENT RECEPTOR POTENTIAL CHANNEL / POLYCYSTIC KIDNEY DISEASE / Structural Genomics / Structural Genomics Consortium / SGC | |||||||||
| Function / homology |  Function and homology information Function and homology informationdetection of nodal flow / metanephric smooth muscle tissue development / metanephric cortex development / metanephric cortical collecting duct development / metanephric distal tubule development / polycystin complex / mesonephric tubule development / mesonephric duct development / metanephric part of ureteric bud development / renal tubule morphogenesis ...detection of nodal flow / metanephric smooth muscle tissue development / metanephric cortex development / metanephric cortical collecting duct development / metanephric distal tubule development / polycystin complex / mesonephric tubule development / mesonephric duct development / metanephric part of ureteric bud development / renal tubule morphogenesis / determination of liver left/right asymmetry / metanephric ascending thin limb development / metanephric mesenchyme development / metanephric S-shaped body morphogenesis / basal cortex / renal artery morphogenesis / HLH domain binding / calcium-induced calcium release activity / VxPx cargo-targeting to cilium / cilium organization / migrasome / muscle alpha-actinin binding / detection of mechanical stimulus / regulation of calcium ion import / voltage-gated monoatomic ion channel activity / placenta blood vessel development / cellular response to hydrostatic pressure / cellular response to fluid shear stress / outward rectifier potassium channel activity / non-motile cilium / cation channel complex / cellular response to osmotic stress / determination of left/right symmetry / actinin binding / : / neural tube development / voltage-gated sodium channel activity / voltage-gated monoatomic cation channel activity / motile cilium / aorta development / ciliary membrane / branching involved in ureteric bud morphogenesis / negative regulation of G1/S transition of mitotic cell cycle / protein heterotetramerization / spinal cord development / positive regulation of phospholipase C-activating G protein-coupled receptor signaling pathway / heart looping / cytoplasmic side of endoplasmic reticulum membrane / centrosome duplication / voltage-gated potassium channel activity / cell surface receptor signaling pathway via JAK-STAT / potassium channel activity / embryonic placenta development / transcription regulator inhibitor activity / voltage-gated calcium channel activity / monoatomic cation channel activity / cytoskeletal protein binding / release of sequestered calcium ion into cytosol / potassium ion transmembrane transport / cellular response to calcium ion / basal plasma membrane / cellular response to cAMP / cytoplasmic vesicle membrane / sodium ion transmembrane transport / cellular response to reactive oxygen species / establishment of localization in cell / lumenal side of endoplasmic reticulum membrane / protein tetramerization / phosphoprotein binding / liver development / calcium ion transmembrane transport / Wnt signaling pathway / intracellular calcium ion homeostasis / positive regulation of nitric oxide biosynthetic process / cell-cell junction / mitotic spindle / calcium ion transport / lamellipodium / regulation of cell population proliferation / heart development / ATPase binding / basolateral plasma membrane / protein homotetramerization / transmembrane transporter binding / cell surface receptor signaling pathway / regulation of cell cycle / cilium / ciliary basal body / signaling receptor binding / negative regulation of cell population proliferation / calcium ion binding / positive regulation of gene expression / endoplasmic reticulum membrane / endoplasmic reticulum / Golgi apparatus / protein homodimerization activity / positive regulation of transcription by RNA polymerase II / extracellular exosome / identical protein binding / membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.96 Å | |||||||||
 Authors Authors | Wang, Q. / Pike, A.C.W. / Grieben, M. / Baronina, A. / Nasrallah, C. / Shintre, C. / Edwards, A.M. / Arrowsmith, C.H. / Bountra, C. / Carpenter, E.P. / Structural Genomics Consortium (SGC) | |||||||||
| Funding support |  United Kingdom, 1items United Kingdom, 1items
| |||||||||
 Citation Citation |  Journal: Structure / Year: 2020 Journal: Structure / Year: 2020Title: Lipid Interactions of a Ciliary Membrane TRP Channel: Simulation and Structural Studies of Polycystin-2. Authors: Qinrui Wang / Robin A Corey / George Hedger / Prafulla Aryal / Mariana Grieben / Chady Nasrallah / Agnese Baronina / Ashley C W Pike / Jiye Shi / Elisabeth P Carpenter / Mark S P Sansom /  Abstract: Polycystin-2 (PC2) is a transient receptor potential (TRP) channel present in ciliary membranes of the kidney. PC2 shares a transmembrane fold with other TRP channels, in addition to an extracellular ...Polycystin-2 (PC2) is a transient receptor potential (TRP) channel present in ciliary membranes of the kidney. PC2 shares a transmembrane fold with other TRP channels, in addition to an extracellular domain found in TRPP and TRPML channels. Using molecular dynamics (MD) simulations and cryoelectron microscopy we identify and characterize PIP and cholesterol interactions with PC2. PC2 is revealed to have a PIP binding site close to the equivalent vanilloid/lipid binding site in the TRPV1 channel. A 3.0-Å structure reveals a binding site for cholesterol on PC2. Cholesterol interactions with the channel at this site are characterized by MD simulations. The two classes of lipid binding sites are compared with sites observed in other TRPs and in Kv channels. These findings suggest PC2, in common with other ion channels, may be modulated by both PIPs and cholesterol, and position PC2 within an emerging model of the roles of lipids in the regulation and organization of ciliary membranes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6t9n.cif.gz 6t9n.cif.gz | 378.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6t9n.ent.gz pdb6t9n.ent.gz | 310.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6t9n.json.gz 6t9n.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/t9/6t9n https://data.pdbj.org/pub/pdb/validation_reports/t9/6t9n ftp://data.pdbj.org/pub/pdb/validation_reports/t9/6t9n ftp://data.pdbj.org/pub/pdb/validation_reports/t9/6t9n | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  10418MC  6t9oC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 1 types, 4 molecules ABCD
| #1: Protein | Mass: 63986.668 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: PKD2, TRPP2 / Plasmid: pFB-CT10HF-LIC / Production host: Homo sapiens (human) / Gene: PKD2, TRPP2 / Plasmid: pFB-CT10HF-LIC / Production host:  |
|---|
-Sugars , 2 types, 12 molecules 
| #2: Polysaccharide | 2-acetamido-2-deoxy-beta-D-glucopyranose-(1-4)-2-acetamido-2-deoxy-beta-D-glucopyranose Source method: isolated from a genetically manipulated source #4: Sugar | ChemComp-NAG / |
|---|
-Non-polymers , 3 types, 37 molecules 
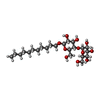



| #3: Chemical | ChemComp-CLR / #5: Chemical | ChemComp-UMQ / #6: Chemical | ChemComp-CA / | |
|---|
-Details
| Has ligand of interest | N |
|---|---|
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: POLYCYSTIN-2 CHANNEL TETRAMER / Type: ORGANELLE OR CELLULAR COMPONENT Details: Detergent (UDM) purified in presence of lipid PI(4,5)P2 Entity ID: #1 / Source: RECOMBINANT | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.256 MDa / Experimental value: NO | |||||||||||||||||||||||||
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||||||||||||
| Source (recombinant) | Organism:  | |||||||||||||||||||||||||
| Buffer solution | pH: 7.5 | |||||||||||||||||||||||||
| Buffer component |
| |||||||||||||||||||||||||
| Specimen | Conc.: 4.5 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | |||||||||||||||||||||||||
| Specimen support | Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R1.2/1.3 | |||||||||||||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 278 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 2500 nm / Nominal defocus min: 1000 nm / Cs: 2.7 mm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Average exposure time: 8 sec. / Electron dose: 52.2 e/Å2 / Detector mode: COUNTING / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Num. of grids imaged: 1 / Num. of real images: 1597 |
| Image scans | Movie frames/image: 20 / Used frames/image: 1-20 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||||||||||
| Image processing | Details: Images were corrected for local motion using patch-based methods with MotionCor2 | ||||||||||||||||||||||||||||||||||||||||
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 98113 | ||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C4 (4 fold cyclic) | ||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.96 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 51694 Details: RELION autorefinement. Unmasked resolution is 3.12. After masking to remove the detergent micelle resolution is 2.96A Num. of class averages: 1 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||
| Atomic model building | B value: 85 / Protocol: AB INITIO MODEL / Space: REAL | ||||||||||||||||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 5K47 Accession code: 5K47 / Source name: PDB / Type: experimental model | ||||||||||||||||||||||||||||||||||||||||
| Refinement | Cross valid method: THROUGHOUT | ||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 93.99 Å2 / Biso mean: 35.0255 Å2 / Biso min: 14.07 Å2 | ||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj











