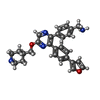
Entry Database : PDB / ID : 6mo0Title Structure of dengue virus protease with an allosteric Inhibitor that blocks replication FLAVIVIRUS_NS2B/Peptidase S7 Keywords / / / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Method / / / Resolution : 2.7 Å Authors Lin, Y.-L. / Nie, S. / Hua, Y. / Wu, J. / Wu, F. / Huo, T. / Yao, Y. / Song, Y. Funding support Organization Grant number Country Other government W81XWH-18-1-0368
Journal : J. Am. Chem. Soc. / Year : 2019Title : Discovery, X-ray Crystallography and Antiviral Activity of Allosteric Inhibitors of Flavivirus NS2B-NS3 Protease.Authors : Yao, Y. / Huo, T. / Lin, Y.L. / Nie, S. / Wu, F. / Hua, Y. / Wu, J. / Kneubehl, A.R. / Vogt, M.B. / Rico-Hesse, R. / Song, Y. History Deposition Oct 3, 2018 Deposition site / Processing site Revision 1.0 May 22, 2019 Provider / Type Revision 1.1 Oct 11, 2023 Group / Database references / Refinement descriptionCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model / struct_ncs_dom_lim Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _struct_ncs_dom_lim.beg_auth_comp_id / _struct_ncs_dom_lim.beg_label_asym_id / _struct_ncs_dom_lim.beg_label_comp_id / _struct_ncs_dom_lim.beg_label_seq_id / _struct_ncs_dom_lim.end_auth_comp_id / _struct_ncs_dom_lim.end_label_asym_id / _struct_ncs_dom_lim.end_label_comp_id / _struct_ncs_dom_lim.end_label_seq_id
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Dengue virus 2
Dengue virus 2 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.7 Å
MOLECULAR REPLACEMENT / Resolution: 2.7 Å  Authors
Authors United States, 1items
United States, 1items  Citation
Citation Journal: J. Am. Chem. Soc. / Year: 2019
Journal: J. Am. Chem. Soc. / Year: 2019 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 6mo0.cif.gz
6mo0.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb6mo0.ent.gz
pdb6mo0.ent.gz PDB format
PDB format 6mo0.json.gz
6mo0.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/mo/6mo0
https://data.pdbj.org/pub/pdb/validation_reports/mo/6mo0 ftp://data.pdbj.org/pub/pdb/validation_reports/mo/6mo0
ftp://data.pdbj.org/pub/pdb/validation_reports/mo/6mo0


 Links
Links Assembly
Assembly


 Components
Components Dengue virus 2 / Production host:
Dengue virus 2 / Production host: 
 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 19-ID / Wavelength: 0.97918 Å
/ Beamline: 19-ID / Wavelength: 0.97918 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller











 PDBj
PDBj