+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6jvk | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of human MTH1 in complex with compound MI1012 | ||||||
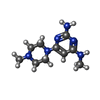
 Components Components | 7,8-dihydro-8-oxoguanine triphosphatase | ||||||
 Keywords Keywords | HYDROLASE / MTH1 / Oxidative DNA damage / 8-oxo-dGTP / Inhibitor development | ||||||
| Function / homology |  Function and homology information Function and homology information2-hydroxy-ATP hydrolase activity / 2-hydroxy-dATP hydrolase activity / N6-methyl-(d)ATP hydrolase activity / O6-methyl-dGTP hydrolase activity / 2-hydroxy-dATP diphosphatase / dATP diphosphatase activity / ATP diphosphatase activity / 8-oxo-7,8-dihydrodeoxyguanosine triphosphate pyrophosphatase activity / 8-oxo-7,8-dihydroguanosine triphosphate pyrophosphatase activity / Phosphate bond hydrolysis by NUDT proteins ...2-hydroxy-ATP hydrolase activity / 2-hydroxy-dATP hydrolase activity / N6-methyl-(d)ATP hydrolase activity / O6-methyl-dGTP hydrolase activity / 2-hydroxy-dATP diphosphatase / dATP diphosphatase activity / ATP diphosphatase activity / 8-oxo-7,8-dihydrodeoxyguanosine triphosphate pyrophosphatase activity / 8-oxo-7,8-dihydroguanosine triphosphate pyrophosphatase activity / Phosphate bond hydrolysis by NUDT proteins / DNA protection / hydrolase activity, acting on acid anhydrides, in phosphorus-containing anhydrides / purine nucleoside catabolic process / snoRNA binding / Hydrolases; Acting on acid anhydrides; In phosphorus-containing anhydrides / response to oxidative stress / mitochondrial matrix / DNA repair / mitochondrion / metal ion binding / nucleus / cytosol / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.1 Å MOLECULAR REPLACEMENT / Resolution: 2.1 Å | ||||||
 Authors Authors | Peng, C. / Li, Y.H. / Cheng, Y.S. | ||||||
 Citation Citation |  Journal: Bioorg.Chem. / Year: 2021 Journal: Bioorg.Chem. / Year: 2021Title: Inhibitor development of MTH1 via high-throughput screening with fragment based library and MTH1 substrate binding cavity. Authors: Peng, C. / Li, Y.H. / Yu, C.W. / Cheng, Z.H. / Liu, J.R. / Hsu, J.L. / Hsin, L.W. / Huang, C.T. / Juan, H.F. / Chern, J.W. / Cheng, Y.S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6jvk.cif.gz 6jvk.cif.gz | 147.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6jvk.ent.gz pdb6jvk.ent.gz | 114.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6jvk.json.gz 6jvk.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jv/6jvk https://data.pdbj.org/pub/pdb/validation_reports/jv/6jvk ftp://data.pdbj.org/pub/pdb/validation_reports/jv/6jvk ftp://data.pdbj.org/pub/pdb/validation_reports/jv/6jvk | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6jvfC  6jvgC  6jvhC  6jviC  6jvjC  6jvlC  6jvmC  6jvnC  6jvoC  6jvpC  6jvqC  6jvrC  6jvsC  6jvtC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||||||||||||||||||||||||||
| 2 | 
| |||||||||||||||||||||||||||||||||
| Unit cell |
| |||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: 1 / Ens-ID: 1 / End auth comp-ID: VAL / End label comp-ID: VAL
|
- Components
Components
| #1: Protein | Mass: 17971.461 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: NUDT1, MTH1 / Plasmid: pET28a / Production host: Homo sapiens (human) / Gene: NUDT1, MTH1 / Plasmid: pET28a / Production host:  References: UniProt: P36639, 8-oxo-dGTP diphosphatase, 2-hydroxy-dATP diphosphatase #2: Chemical | ChemComp-CEU / | #3: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.26 Å3/Da / Density % sol: 45.5 % / Mosaicity: 0.376 ° |
|---|---|
| Crystal grow | Temperature: 298 K / Method: evaporation / pH: 3.75 Details: 30% PEG6000, 200 mM Lithium sulphate, 100 mM Sodium acetate pH 3.75 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  NSRRC NSRRC  / Beamline: BL13B1 / Wavelength: 1 Å / Beamline: BL13B1 / Wavelength: 1 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: RAYONIX MX300HE / Detector: CCD / Date: Oct 2, 2018 / Details: mirrors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 2.1→30 Å / Num. obs: 19183 / % possible obs: 99.7 % / Redundancy: 4.6 % / Biso Wilson estimate: 22.83 Å2 / Rmerge(I) obs: 0.066 / Rpim(I) all: 0.035 / Rrim(I) all: 0.075 / Χ2: 1.054 / Net I/σ(I): 11.4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 2.1→23.706 Å / SU ML: 0.28 / Cross valid method: THROUGHOUT / σ(F): 1.35 / Phase error: 25.14 MOLECULAR REPLACEMENT / Resolution: 2.1→23.706 Å / SU ML: 0.28 / Cross valid method: THROUGHOUT / σ(F): 1.35 / Phase error: 25.14
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 87.34 Å2 / Biso mean: 27.003 Å2 / Biso min: 11.68 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.1→23.706 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints NCS |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0 / Total num. of bins used: 14
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: -13.5207 Å / Origin y: -0.5829 Å / Origin z: 10.1464 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: all |
 Movie
Movie Controller
Controller
















 PDBj
PDBj