Entry Database : PDB / ID : 5czbTitle HCV NS5B IN COMPLEX WITH LIGAND IDX17119-5 NS5B Keywords / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Method / / / Resolution : 1.96 Å Authors Pierra, C. / Dousson, C. / Augustin, M. Journal : Bioorg.Med.Chem.Lett. / Year : 2016Title : Synthesis of potent and broad genotypically active NS5B HCV non-nucleoside inhibitors binding to the thumb domain allosteric site 2 of the viral polymerase.Authors: Pierra Rouviere, C. / Amador, A. / Badaroux, E. / Convard, T. / Da Costa, D. / Dukhan, D. / Griffe, L. / Griffon, J.F. / LaColla, M. / Leroy, F. / Liuzzi, M. / Loi, A.G. / McCarville, J. / ... Authors : Pierra Rouviere, C. / Amador, A. / Badaroux, E. / Convard, T. / Da Costa, D. / Dukhan, D. / Griffe, L. / Griffon, J.F. / LaColla, M. / Leroy, F. / Liuzzi, M. / Loi, A.G. / McCarville, J. / Mascia, V. / Milhau, J. / Onidi, L. / Paparin, J.L. / Rahali, R. / Sais, E. / Seifer, M. / Surleraux, D. / Standring, D. / Dousson, C. History Deposition Jul 31, 2015 Deposition site / Processing site Revision 1.0 Jun 15, 2016 Provider / Type Revision 1.1 Aug 24, 2016 Group Revision 1.2 Sep 7, 2016 Group Revision 1.3 May 1, 2024 Group / Database references / Refinement descriptionCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model Item / _database_2.pdbx_database_accession
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information Hepatitis C virus subtype 1b
Hepatitis C virus subtype 1b X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.96 Å
MOLECULAR REPLACEMENT / Resolution: 1.96 Å  Authors
Authors Citation
Citation Journal: Bioorg.Med.Chem.Lett. / Year: 2016
Journal: Bioorg.Med.Chem.Lett. / Year: 2016 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 5czb.cif.gz
5czb.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb5czb.ent.gz
pdb5czb.ent.gz PDB format
PDB format 5czb.json.gz
5czb.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads 5czb_validation.pdf.gz
5czb_validation.pdf.gz wwPDB validaton report
wwPDB validaton report 5czb_full_validation.pdf.gz
5czb_full_validation.pdf.gz 5czb_validation.xml.gz
5czb_validation.xml.gz 5czb_validation.cif.gz
5czb_validation.cif.gz https://data.pdbj.org/pub/pdb/validation_reports/cz/5czb
https://data.pdbj.org/pub/pdb/validation_reports/cz/5czb ftp://data.pdbj.org/pub/pdb/validation_reports/cz/5czb
ftp://data.pdbj.org/pub/pdb/validation_reports/cz/5czb Links
Links Assembly
Assembly


 Components
Components Hepatitis C virus subtype 1b / Production host:
Hepatitis C virus subtype 1b / Production host: 
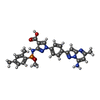










 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  ESRF
ESRF  / Beamline: ID29 / Wavelength: 0.97244 Å
/ Beamline: ID29 / Wavelength: 0.97244 Å Processing
Processing MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller























 PDBj
PDBj




