[English] 日本語
 Yorodumi
Yorodumi- PDB-4aj1: rat LDHA in complex with N-(2-(methylamino)-1,3-benzothiazol-6-yl... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4aj1 | ||||||
|---|---|---|---|---|---|---|---|
| Title | rat LDHA in complex with N-(2-(methylamino)-1,3-benzothiazol-6-yl) acetamide | ||||||
 Components Components | L-LACTATE DEHYDROGENASE A CHAIN | ||||||
 Keywords Keywords | OXIDOREDUCTASE/INHIBITOR / OXIDOREDUCTASE-INHIBITOR COMPLEX / FRAGMENT BASED LEAD GENERATED INHIBITORS | ||||||
| Function / homology |  Function and homology information Function and homology informationlactate dehydrogenase activity / Pyruvate metabolism / Regulation of pyruvate metabolism / sperm fibrous sheath / pyruvate catabolic process / L-lactate dehydrogenase / cellular response to phorbol 13-acetate 12-myristate / oxidoreductase complex / pyruvate metabolic process / NAD+ metabolic process ...lactate dehydrogenase activity / Pyruvate metabolism / Regulation of pyruvate metabolism / sperm fibrous sheath / pyruvate catabolic process / L-lactate dehydrogenase / cellular response to phorbol 13-acetate 12-myristate / oxidoreductase complex / pyruvate metabolic process / NAD+ metabolic process / L-lactate dehydrogenase (NAD+) activity / lactate metabolic process / homolactic fermentation / response to cAMP / response to glucose / skeletal muscle tissue development / response to nutrient / response to hydrogen peroxide / liver development / response to estrogen / kinase binding / NAD binding / response to hypoxia / positive regulation of apoptotic process / response to xenobiotic stimulus / mitochondrion / identical protein binding / cytosol Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.87 Å MOLECULAR REPLACEMENT / Resolution: 1.87 Å | ||||||
 Authors Authors | Tucker, J.A. / Brassington, C. / Hassall, G. / Vogtherr, M. / Ward, R. / Tart, J. / Davies, G. | ||||||
 Citation Citation |  Journal: J.Med.Chem. / Year: 2012 Journal: J.Med.Chem. / Year: 2012Title: The Design and Synthesis of Novel Lactate Dehydrogenase a Inhibitors by Fragment-Based Lead Generation Authors: Ward, R. / Brassington, C. / Breeze, A.L. / Caputo, A. / Critchlow, S. / Davies, G. / Goodwin, L. / Hassall, G. / Greenwood, R. / Holdgate, G. / Mrosek, M. / Norman, R.A. / Pearson, S. / ...Authors: Ward, R. / Brassington, C. / Breeze, A.L. / Caputo, A. / Critchlow, S. / Davies, G. / Goodwin, L. / Hassall, G. / Greenwood, R. / Holdgate, G. / Mrosek, M. / Norman, R.A. / Pearson, S. / Tart, J. / Tucker, J.A. / Vogtherr, M. / Whittaker, D. / Wingfield, J. / Winter, J. / Hudson, K. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4aj1.cif.gz 4aj1.cif.gz | 515.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4aj1.ent.gz pdb4aj1.ent.gz | 427.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4aj1.json.gz 4aj1.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  4aj1_validation.pdf.gz 4aj1_validation.pdf.gz | 507.9 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  4aj1_full_validation.pdf.gz 4aj1_full_validation.pdf.gz | 523.1 KB | Display | |
| Data in XML |  4aj1_validation.xml.gz 4aj1_validation.xml.gz | 59.8 KB | Display | |
| Data in CIF |  4aj1_validation.cif.gz 4aj1_validation.cif.gz | 87 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/aj/4aj1 https://data.pdbj.org/pub/pdb/validation_reports/aj/4aj1 ftp://data.pdbj.org/pub/pdb/validation_reports/aj/4aj1 ftp://data.pdbj.org/pub/pdb/validation_reports/aj/4aj1 | HTTPS FTP |
-Related structure data
| Related structure data |  4aj2C  4aj4C  4ajeC  4ajhC  4ajiC  4ajjC  4ajkC  4ajlC  4ajnC  4ajoC  4ajpC  4al4C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||||||
| Unit cell |
| ||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS oper:
|
- Components
Components
-Protein , 1 types, 4 molecules ABCD
| #1: Protein | Mass: 36363.238 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|
-Non-polymers , 5 types, 1042 molecules 
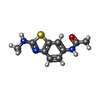







| #2: Chemical | ChemComp-GOL / #3: Chemical | ChemComp-AJ1 / #4: Chemical | ChemComp-MLI / #5: Chemical | ChemComp-OXM / | #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.32 Å3/Da / Density % sol: 47 % / Description: NONE |
|---|---|
| Crystal grow | pH: 7 / Details: 1.5 M NA MALONATE PH 7.0, 2% GLYCEROL |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU FR-E / Wavelength: 1.54 ROTATING ANODE / Type: RIGAKU FR-E / Wavelength: 1.54 |
| Detector | Type: RIGAKU SATURN 944 / Detector: CCD / Date: Dec 8, 2008 / Details: VARIMAXHF |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.54 Å / Relative weight: 1 |
| Reflection | Resolution: 1.87→64.06 Å / Num. obs: 78548 / % possible obs: 73.8 % / Observed criterion σ(I): 2 / Redundancy: 2.81 % / Rmerge(I) obs: 0.07 / Net I/σ(I): 8.3 |
| Reflection shell | Resolution: 1.87→1.94 Å / Redundancy: 1.8 % / Rmerge(I) obs: 0.27 / Mean I/σ(I) obs: 2.2 / % possible all: 33.7 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: INTERNAL RAT LDHA STRUCTURE Resolution: 1.87→49.37 Å / Cor.coef. Fo:Fc: 0.962 / Cor.coef. Fo:Fc free: 0.936 / SU B: 6.652 / SU ML: 0.105 / Cross valid method: THROUGHOUT / ESU R: 0.232 / ESU R Free: 0.191 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS. U VALUES WITH TLS ADDED. DISORDERED SIDE-CHAINS HAVE BEEN TRUNCATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: BABINET MODEL WITH MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 22.782 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.87→49.37 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj





