+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of phage DEV ejection proteins gp72:gp73 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | phage / bacteriophage / STRUCTURAL PROTEIN / VIRAL PROTEIN / outer membrane protein / gp73 / gp74 / DEV | |||||||||
| Function / homology | Uncharacterized protein / N4 gp52-like protein Function and homology information Function and homology information | |||||||||
| Biological species |  Pseudomonas phage vB_PaeP_DEV (virus) Pseudomonas phage vB_PaeP_DEV (virus) | |||||||||
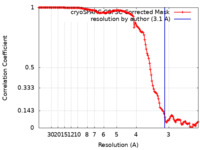
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Iglesias SM / Cingolani G | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation | Journal: Res Sq / Year: 2024 Title: Integrative structural analysis of phage DEV reveals a genome ejection motor. Authors: Gino Cingolani / Ravi Lokareddy / Chun-Feng Hou / Francesca Forti / Stephano Iglesias / Fenglin Li / Mikhail Pavlenok / Michael Niederweis / Federica Briani Abstract: DEV is an obligatory lytic phage of the N4-like genus, recently reclassified as . The DEV genome encodes 91 ORFs, including a 3,398 amino acid virion-associated RNA polymerase. Here, we describe the ...DEV is an obligatory lytic phage of the N4-like genus, recently reclassified as . The DEV genome encodes 91 ORFs, including a 3,398 amino acid virion-associated RNA polymerase. Here, we describe the complete architecture of DEV, determined using a combination of cryo-electron microscopy localized reconstruction, biochemical methods, and genetic knockouts. We built de structures of all capsid factors and tail components involved in host attachment. We demonstrate that DEV long tail fibers are essential for infection of and dispensable for infecting mutants with a truncated lipopolysaccharide devoid of the O-antigen. We identified DEV ejection proteins and, unexpectedly, found that the giant DEV RNA polymerase, the hallmark of the family, is an ejection protein. We propose that DEV ejection proteins form a genome ejection motor across the host cell envelope and that these structural principles are conserved in all . | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_43629.map.gz emd_43629.map.gz | 228.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-43629-v30.xml emd-43629-v30.xml emd-43629.xml emd-43629.xml | 17.6 KB 17.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_43629_fsc.xml emd_43629_fsc.xml | 13.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_43629.png emd_43629.png | 35.9 KB | ||
| Masks |  emd_43629_msk_1.map emd_43629_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-43629.cif.gz emd-43629.cif.gz | 6.1 KB | ||
| Others |  emd_43629_half_map_1.map.gz emd_43629_half_map_1.map.gz emd_43629_half_map_2.map.gz emd_43629_half_map_2.map.gz | 221.6 MB 221.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-43629 http://ftp.pdbj.org/pub/emdb/structures/EMD-43629 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43629 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-43629 | HTTPS FTP |
-Validation report
| Summary document |  emd_43629_validation.pdf.gz emd_43629_validation.pdf.gz | 721.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_43629_full_validation.pdf.gz emd_43629_full_validation.pdf.gz | 720.9 KB | Display | |
| Data in XML |  emd_43629_validation.xml.gz emd_43629_validation.xml.gz | 22.2 KB | Display | |
| Data in CIF |  emd_43629_validation.cif.gz emd_43629_validation.cif.gz | 28.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43629 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43629 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43629 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-43629 | HTTPS FTP |
-Related structure data
| Related structure data |  8vxqMC  9bgmC  9bgnC  9bgoC  9codC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_43629.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_43629.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.21 Å | ||||||||||||||||||||||||||||||||||||
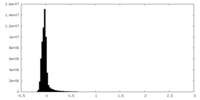
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_43629_msk_1.map emd_43629_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
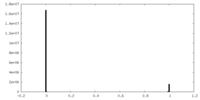
| Density Histograms |
-Half map: #2
| File | emd_43629_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
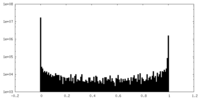
| Density Histograms |
-Half map: #1
| File | emd_43629_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
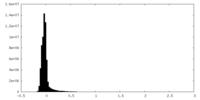
| Density Histograms |
- Sample components
Sample components
-Entire : Pseudomonas phage vB_PaeP_DEV
| Entire | Name:  Pseudomonas phage vB_PaeP_DEV (virus) Pseudomonas phage vB_PaeP_DEV (virus) |
|---|---|
| Components |
|
-Supramolecule #1: Pseudomonas phage vB_PaeP_DEV
| Supramolecule | Name: Pseudomonas phage vB_PaeP_DEV / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 2034344 / Sci species name: Pseudomonas phage vB_PaeP_DEV / Virus type: VIRION / Virus isolate: OTHER / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:  |
-Macromolecule #1: gp72
| Macromolecule | Name: gp72 / type: protein_or_peptide / ID: 1 / Number of copies: 9 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudomonas phage vB_PaeP_DEV (virus) Pseudomonas phage vB_PaeP_DEV (virus) |
| Molecular weight | Theoretical: 57.183238 KDa |
| Sequence | String: MAQEITWRNI GATVSPGSAS SMSAGTTGVQ QALGALGDII SRQQEMNVNN AKLQREANTQ SYLDQVAAST LEQLSNADYR SGLEAQRDA MGMNLDRAAT RDAITKQISA QQNQAAATQK FDDMQAEVGQ RGIVDQLRTL SAEGRAGEVN QILAEQQLIN E GEIRKELT ...String: MAQEITWRNI GATVSPGSAS SMSAGTTGVQ QALGALGDII SRQQEMNVNN AKLQREANTQ SYLDQVAAST LEQLSNADYR SGLEAQRDA MGMNLDRAAT RDAITKQISA QQNQAAATQK FDDMQAEVGQ RGIVDQLRTL SAEGRAGEVN QILAEQQLIN E GEIRKELT GVQDAIQNRQ YRAAGEQRAQ AAANRAAEAH SLSMAAGREN LAFTREQRDE LRRDRDEAKL VSGTIATTFQ DY DESRQAQ SEIMRIVGKE VGMPTDDQGM PDMSRASQDQ LDAFSNALNE AGVQANTSPT ERRNAVLKSL VDAGVSSKGI AQA KQEMEL RESLEGLAPQ DRTKVEATIG AVNAELDTLQ RTATEDYERE VARNPFVEPD KDPLGSVNKI VDKAVKSGFG WEGD RQDLN NMLVDFATNG IKLPDGRTAV VPSKLLEQAF NTTNTWLFKN AGDVEKRIIE LMTTDGMTQM REDAPTIREN FLKTV SDIA NQKRSNAVKV TRSAEREKGV TMDPTDDLTF ALRGRKR UniProtKB: Uncharacterized protein |
-Macromolecule #2: N4 gp52-like protein
| Macromolecule | Name: N4 gp52-like protein / type: protein_or_peptide / ID: 2 / Number of copies: 9 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Pseudomonas phage vB_PaeP_DEV (virus) Pseudomonas phage vB_PaeP_DEV (virus) |
| Molecular weight | Theoretical: 16.639414 KDa |
| Sequence | String: MAYPYSDMPF GVELDTSTLG SFGLGGPQTQ LQMQMPAVDV NAAASGSGGF MAGFSNIFSR DSMFGGVAPS GAQTGGWVLP ALGIGQAVF GAIGANRQQR AARDQLAESR RQFDMNYGAQ RQSINTNLED RQRARVASNP TAYESVDSYM ERNRIR UniProtKB: N4 gp52-like protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 15 sec. / Pretreatment - Atmosphere: OTHER |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 1.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: OTHER / Imaging mode: OTHER / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.8 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller








 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)