+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3654 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of an ABC transporter | |||||||||
 Map data Map data | Map after post-processing and masking | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ABC transporter / transport protein | |||||||||
| Function / homology |  Function and homology information Function and homology informationbiotin transmembrane transporter activity / biotin transport / riboflavin transport / riboflavin transmembrane transporter activity / sphingolipid transporter activity / renal urate salt excretion / Abacavir transmembrane transport / urate metabolic process / sphingolipid biosynthetic process / Sphingolipid de novo biosynthesis ...biotin transmembrane transporter activity / biotin transport / riboflavin transport / riboflavin transmembrane transporter activity / sphingolipid transporter activity / renal urate salt excretion / Abacavir transmembrane transport / urate metabolic process / sphingolipid biosynthetic process / Sphingolipid de novo biosynthesis / urate transmembrane transporter activity / external side of apical plasma membrane / xenobiotic transport across blood-brain barrier / organic anion transport / : / transepithelial transport / Ciprofloxacin ADME / Paracetamol ADME / export across plasma membrane / NFE2L2 regulating MDR associated enzymes / Differentiation of Keratinocytes in Interfollicular Epidermis in Mammalian Skin / ABC-type xenobiotic transporter / Heme biosynthesis / cellular detoxification / ABC-type xenobiotic transporter activity / Heme degradation / efflux transmembrane transporter activity / ATPase-coupled transmembrane transporter activity / xenobiotic transmembrane transporter activity / transport across blood-brain barrier / brush border membrane / Iron uptake and transport / transmembrane transport / mitochondrial membrane / apical plasma membrane / membrane raft / protein homodimerization activity / ATP hydrolysis activity / nucleoplasm / ATP binding / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
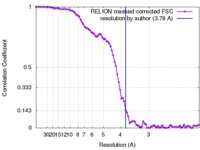
| Method | single particle reconstruction / cryo EM / Resolution: 3.78 Å | |||||||||
 Authors Authors | Taylor NMI / Manolaridis I | |||||||||
| Funding support |  Switzerland, 2 items Switzerland, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2017 Journal: Nature / Year: 2017Title: Structure of the human multidrug transporter ABCG2. Authors: Nicholas M I Taylor / Ioannis Manolaridis / Scott M Jackson / Julia Kowal / Henning Stahlberg / Kaspar P Locher /  Abstract: ABCG2 is a constitutively expressed ATP-binding cassette (ABC) transporter that protects many tissues against xenobiotic molecules. Its activity affects the pharmacokinetics of commonly used drugs ...ABCG2 is a constitutively expressed ATP-binding cassette (ABC) transporter that protects many tissues against xenobiotic molecules. Its activity affects the pharmacokinetics of commonly used drugs and limits the delivery of therapeutics into tumour cells, thus contributing to multidrug resistance. Here we present the structure of human ABCG2 determined by cryo-electron microscopy, providing the first high-resolution insight into a human multidrug transporter. We visualize ABCG2 in complex with two antigen-binding fragments of the human-specific, inhibitory antibody 5D3 that recognizes extracellular loops of the transporter. We observe two cholesterol molecules bound in the multidrug-binding pocket that is located in a central, hydrophobic, inward-facing translocation pathway between the transmembrane domains. Combined with functional in vitro analyses, our results suggest a multidrug recognition and transport mechanism of ABCG2, rationalize disease-causing single nucleotide polymorphisms and the allosteric inhibition by the 5D3 antibody, and provide the structural basis of cholesterol recognition by other G-subfamily ABC transporters. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3654.map.gz emd_3654.map.gz | 6.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3654-v30.xml emd-3654-v30.xml emd-3654.xml emd-3654.xml | 14.4 KB 14.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_3654_fsc.xml emd_3654_fsc.xml | 8.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_3654.png emd_3654.png | 56.9 KB | ||
| Filedesc metadata |  emd-3654.cif.gz emd-3654.cif.gz | 6.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3654 http://ftp.pdbj.org/pub/emdb/structures/EMD-3654 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3654 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3654 | HTTPS FTP |
-Related structure data
| Related structure data |  5nj3MC  5njgMC  5nivC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_3654.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3654.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map after post-processing and masking | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.039 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Nanodisc-reconstituted ABCG2 in complex with 5D3-Fab
| Entire | Name: Nanodisc-reconstituted ABCG2 in complex with 5D3-Fab |
|---|---|
| Components |
|
-Supramolecule #1: Nanodisc-reconstituted ABCG2 in complex with 5D3-Fab
| Supramolecule | Name: Nanodisc-reconstituted ABCG2 in complex with 5D3-Fab / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|
-Supramolecule #2: Nanodisc-reconstituted ABCG2
| Supramolecule | Name: Nanodisc-reconstituted ABCG2 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: 5D3-Fab
| Supramolecule | Name: 5D3-Fab / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2-#3 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: ATP-binding cassette sub-family G member 2
| Macromolecule | Name: ATP-binding cassette sub-family G member 2 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 73.395742 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: DYKDDDDKGS SSSNVEVFIP VSQGNTNGFP ATASNDLKAF TEGAVLSFHN ICYRVKLKSG FLPCRKPVEK EILSNINGIM KPGLNAILG PTGGGKSSLL DVLAARKDPS GLSGDVLING APRPANFKCN SGYVVQDDVV MGTLTVRENL QFSAALRLAT T MTNHEKNE ...String: DYKDDDDKGS SSSNVEVFIP VSQGNTNGFP ATASNDLKAF TEGAVLSFHN ICYRVKLKSG FLPCRKPVEK EILSNINGIM KPGLNAILG PTGGGKSSLL DVLAARKDPS GLSGDVLING APRPANFKCN SGYVVQDDVV MGTLTVRENL QFSAALRLAT T MTNHEKNE RINRVIQELG LDKVADSKVG TQFIRGVSGG ERKRTSIGME LITDPSILFL DEPTTGLDSS TANAVLLLLK RM SKQGRTI IFSIHQPRYS IFKLFDSLTL LASGRLMFHG PAQEALGYFE SAGYHCEAYN NPADFFLDII NGDSTAVALN REE DFKATE IIEPSKQDKP LIEKLAEIYV NSSFYKETKA ELHQLSGGEK KKKITVFKEI SYTTSFCHQL RWVSKRSFKN LLGN PQASI AQIIVTVVLG LVIGAIYFGL KNDSTGIQNR AGVLFFLTTN QCFSSVSAVE LFVVEKKLFI HEYISGYYRV SSYFL GKLL SDLLPMRMLP SIIFTCIVYF MLGLKPKADA FFVMMFTLMM VAYSASSMAL AIAAGQSVVS VATLLMTICF VFMMIF SGL LVNLTTIASW LSWLQYFSIP RYGFTALQHN EFLGQNFCPG LNATGNNPCN YATCTGEEYL VKQGIDLSPW GLWKNHV AL ACMIVIFLTI AYLKLLFLKK YS UniProtKB: Broad substrate specificity ATP-binding cassette transporter ABCG2 |
-Macromolecule #2: 5D3-Fab heavy chain
| Macromolecule | Name: 5D3-Fab heavy chain / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 23.843633 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLQESGPG LVKPSQSLSL TCTVTGFSIT SDYAWNWIRQ FPGKKLEWMG YINFDGGTTY NPSLRGRISI TRDTSKNQFF LQLRSVTPE DTATYYCATF YGAKGTLDYW GQGTSVTVSS AKTTPPSVYP LAPVCGDTSG SSVTLGCLVK GYFPEPVTLT W NSGSLSSG ...String: QVQLQESGPG LVKPSQSLSL TCTVTGFSIT SDYAWNWIRQ FPGKKLEWMG YINFDGGTTY NPSLRGRISI TRDTSKNQFF LQLRSVTPE DTATYYCATF YGAKGTLDYW GQGTSVTVSS AKTTPPSVYP LAPVCGDTSG SSVTLGCLVK GYFPEPVTLT W NSGSLSSG VHTFPAVLQS DLYTLSSSVT VTSSTWPSQS ITCNVAHPAS STKVDKKIEP RGP |
-Macromolecule #3: 5D3-Fab light chain
| Macromolecule | Name: 5D3-Fab light chain / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 23.594016 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: DIVLTQSPSS FSVSLGDRVT ISCKASGYIL NRLAWYQQKP GNAPRLLISG ATSLETGFPS RFSGTGSGKD YTLSISSLQT EDVGTYYCQ QYWSTPWTFG GGTKLEIRRA DAAPTVSIFP PSSEQLTSGG ASVVCFLNNF YPKDINVKWK IDGSERQNGV L NSWTDQDS ...String: DIVLTQSPSS FSVSLGDRVT ISCKASGYIL NRLAWYQQKP GNAPRLLISG ATSLETGFPS RFSGTGSGKD YTLSISSLQT EDVGTYYCQ QYWSTPWTFG GGTKLEIRRA DAAPTVSIFP PSSEQLTSGG ASVVCFLNNF YPKDINVKWK IDGSERQNGV L NSWTDQDS KDSTYSMSST LTLTKDEYER HNSYTCEATH KTSTSPIVKS FNRNEC |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Frames/image: 1-40 / Average electron dose: 67.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)