+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of a GPCR-Gi complex with peptide | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationmesendoderm migration / mesoderm migration involved in gastrulation / cell migration involved in mesendoderm migration / apelin receptor binding / apelin receptor activity / apelin receptor signaling pathway / regulation of gap junction assembly / positive regulation of G protein-coupled receptor internalization / vascular associated smooth muscle cell differentiation / atrioventricular valve development ...mesendoderm migration / mesoderm migration involved in gastrulation / cell migration involved in mesendoderm migration / apelin receptor binding / apelin receptor activity / apelin receptor signaling pathway / regulation of gap junction assembly / positive regulation of G protein-coupled receptor internalization / vascular associated smooth muscle cell differentiation / atrioventricular valve development / regulation of body fluid levels / venous blood vessel development / positive regulation of heart contraction / positive regulation of trophoblast cell migration / positive regulation of blood vessel endothelial cell proliferation involved in sprouting angiogenesis / endocardial cushion formation / placenta blood vessel development / negative regulation of cAMP-mediated signaling / endoderm development / coronary vasculature development / adult heart development / vasculature development / embryonic heart tube development / aorta development / ventricular septum morphogenesis / blood vessel development / heart looping / T cell migration / D2 dopamine receptor binding / vasculogenesis / Adenylate cyclase inhibitory pathway / positive regulation of protein localization to cell cortex / regulation of cAMP-mediated signaling / G protein-coupled serotonin receptor binding / cellular response to forskolin / gastrulation / regulation of mitotic spindle organization / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / Peptide ligand-binding receptors / positive regulation of release of sequestered calcium ion into cytosol / Regulation of insulin secretion / G protein-coupled receptor binding / G protein-coupled receptor activity / electron transport chain / G-protein beta/gamma-subunit complex binding / Olfactory Signaling Pathway / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / Activation of the phototransduction cascade / hormone activity / G beta:gamma signalling through PLC beta / Presynaptic function of Kainate receptors / Thromboxane signalling through TP receptor / G protein-coupled acetylcholine receptor signaling pathway / G-protein activation / Activation of G protein gated Potassium channels / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Prostacyclin signalling through prostacyclin receptor / response to peptide hormone / Glucagon signaling in metabolic regulation / G beta:gamma signalling through CDC42 / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Sensory perception of sweet, bitter, and umami (glutamate) taste / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / photoreceptor disc membrane / Glucagon-type ligand receptors / Adrenaline,noradrenaline inhibits insulin secretion / Vasopressin regulates renal water homeostasis via Aquaporins / G alpha (z) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / cellular response to catecholamine stimulus / ADORA2B mediated anti-inflammatory cytokines production / positive regulation of angiogenesis / sensory perception of taste / ADP signalling through P2Y purinoceptor 1 / G beta:gamma signalling through PI3Kgamma / adenylate cyclase-activating dopamine receptor signaling pathway / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / GPER1 signaling / GDP binding / cellular response to prostaglandin E stimulus / Inactivation, recovery and regulation of the phototransduction cascade / G-protein beta-subunit binding / heterotrimeric G-protein complex / G alpha (12/13) signalling events / extracellular vesicle / signaling receptor complex adaptor activity / Thrombin signalling through proteinase activated receptors (PARs) / GTPase binding / heart development / retina development in camera-type eye / signaling receptor activity / Ca2+ pathway / cell cortex / phospholipase C-activating G protein-coupled receptor signaling pathway / midbody / G alpha (i) signalling events / fibroblast proliferation / regulation of gene expression / G alpha (s) signalling events Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.16 Å | |||||||||
 Authors Authors | Xu F / Yue Y / Liu LE / Wu LJ / Hanson M | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2022 Journal: Nat Struct Mol Biol / Year: 2022Title: Structural insight into apelin receptor-G protein stoichiometry. Authors: Yang Yue / Lier Liu / Li-Jie Wu / Yiran Wu / Ling Wang / Fei Li / Junlin Liu / Gye-Won Han / Bo Chen / Xi Lin / Rebecca L Brouillette / Émile Breault / Jean-Michel Longpré / Songting Shi / ...Authors: Yang Yue / Lier Liu / Li-Jie Wu / Yiran Wu / Ling Wang / Fei Li / Junlin Liu / Gye-Won Han / Bo Chen / Xi Lin / Rebecca L Brouillette / Émile Breault / Jean-Michel Longpré / Songting Shi / Hui Lei / Philippe Sarret / Raymond C Stevens / Michael A Hanson / Fei Xu /    Abstract: The technique of cryogenic-electron microscopy (cryo-EM) has revolutionized the field of membrane protein structure and function with a focus on the dominantly observed molecular species. This report ...The technique of cryogenic-electron microscopy (cryo-EM) has revolutionized the field of membrane protein structure and function with a focus on the dominantly observed molecular species. This report describes the structural characterization of a fully active human apelin receptor (APJR) complexed with heterotrimeric G protein observed in both 2:1 and 1:1 stoichiometric ratios. We use cryo-EM single-particle analysis to determine the structural details of both species from the same sample preparation. Protein preparations, in the presence of the endogenous peptide ligand ELA or a synthetic small molecule, both demonstrate these mixed stoichiometric states. Structural differences in G protein engagement between dimeric and monomeric APJR suggest a role for the stoichiometry of G protein-coupled receptor- (GPCR-)G protein coupling on downstream signaling and receptor pharmacology. Furthermore, a small, hydrophobic dimer interface provides a starting framework for additional class A GPCR dimerization studies. Together, these findings uncover a mechanism of versatile regulation through oligomerization by which GPCRs can modulate their signaling. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32247.map.gz emd_32247.map.gz | 56.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32247-v30.xml emd-32247-v30.xml emd-32247.xml emd-32247.xml | 21.8 KB 21.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_32247.png emd_32247.png | 28.2 KB | ||
| Others |  emd_32247_additional_1.map.gz emd_32247_additional_1.map.gz emd_32247_half_map_1.map.gz emd_32247_half_map_1.map.gz emd_32247_half_map_2.map.gz emd_32247_half_map_2.map.gz | 32.2 MB 59.5 MB 59.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32247 http://ftp.pdbj.org/pub/emdb/structures/EMD-32247 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32247 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32247 | HTTPS FTP |
-Validation report
| Summary document |  emd_32247_validation.pdf.gz emd_32247_validation.pdf.gz | 687 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_32247_full_validation.pdf.gz emd_32247_full_validation.pdf.gz | 686.5 KB | Display | |
| Data in XML |  emd_32247_validation.xml.gz emd_32247_validation.xml.gz | 12 KB | Display | |
| Data in CIF |  emd_32247_validation.cif.gz emd_32247_validation.cif.gz | 14 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32247 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32247 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32247 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32247 | HTTPS FTP |
-Related structure data
| Related structure data |  7w0pMC  7susC  7w0lC  7w0mC  7w0nC  7w0oC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32247.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32247.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_32247_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
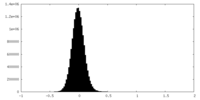
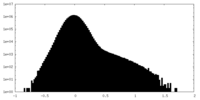
| Density Histograms |
-Half map: #2
| File | emd_32247_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_32247_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : GPCR-Gi Complex
| Entire | Name: GPCR-Gi Complex |
|---|---|
| Components |
|
-Supramolecule #1: GPCR-Gi Complex
| Supramolecule | Name: GPCR-Gi Complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
-Macromolecule #1: Guanine nucleotide-binding protein G(i) subunit alpha-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(i) subunit alpha-1 type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 40.55916 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: GSMGCTLSAE DKAAVERSKM IDRNLREDGE KAAREVKLLL LGAGESGKST IVKQMKIIHE AGYSEEECKQ YKAVVYSNTI QSIIAIIRA MGRLKIDFGD SARADDARQL FVLAGAAEEG FMTAELAGVI KRLWKDSGVQ ACFNRSREYQ LNDSAAYYLN D LDRIAQPN ...String: GSMGCTLSAE DKAAVERSKM IDRNLREDGE KAAREVKLLL LGAGESGKST IVKQMKIIHE AGYSEEECKQ YKAVVYSNTI QSIIAIIRA MGRLKIDFGD SARADDARQL FVLAGAAEEG FMTAELAGVI KRLWKDSGVQ ACFNRSREYQ LNDSAAYYLN D LDRIAQPN YIPTQQDVLR TRVKTTGIVE THFTFKDLHF KMFDVGGQRS ERKKWIHCFE GVTAIIFCVA LSDYDLVLAE DE EMNRMHE SMKLFDSICN NKWFTDTSII LFLNKKDLFE EKIKKSPLTI CYPEYAGSNT YEEAAAYIQC QFEDLNKRKD TKE IYTHFT CATDTKNVQF VFDAVTDVII KNNLKDCGLF |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 37.41693 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL ...String: MSELDQLRQE AEQLKNQIRD ARKACADATL SQITNNIDPV GRIQMRTRRT LRGHLAKIYA MHWGTDSRLL VSASQDGKLI IWDSYTTNK VHAIPLRSSW VMTCAYAPSG NYVACGGLDN ICSIYNLKTR EGNVRVSREL AGHTGYLSCC RFLDDNQIVT S SGDTTCAL WDIETGQQTT TFTGHTGDVM SLSLAPDTRL FVSGACDASA KLWDVREGMC RQTFTGHESD INAICFFPNG NA FATGSDD ATCRLFDLRA DQELMTYSHD NIICGITSVS FSKSGRLLLA GYDDFNCNVW DALKADRAGV LAGHDNRVSC LGV TDDGMA VATGSWDSFL KIWN |
-Macromolecule #3: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L |
-Macromolecule #4: Soluble cytochrome b562,Apelin receptor
| Macromolecule | Name: Soluble cytochrome b562,Apelin receptor / type: protein_or_peptide / ID: 4 Details: Fusion protein of Soluble cytochrome b562, linker and Apelin receptor Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 54.23648 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MKTIIALSYI FCLVFADYKD DDDKHHHHHH HHHHLEVLFQ GPADLEDNWE TLNDNLKVIE KADNAAQVKD ALTKMRAAAL DAQKATPPK LEDKSPDSPE MKDFRHGFDI LVGQIDDALK LANEGKVKEA QAAAEQLKTT RNAYIQKYLE EGGDFDNYYG A DNQSECEY ...String: MKTIIALSYI FCLVFADYKD DDDKHHHHHH HHHHLEVLFQ GPADLEDNWE TLNDNLKVIE KADNAAQVKD ALTKMRAAAL DAQKATPPK LEDKSPDSPE MKDFRHGFDI LVGQIDDALK LANEGKVKEA QAAAEQLKTT RNAYIQKYLE EGGDFDNYYG A DNQSECEY TDWKSSGALI PAIYMLVFLL GTTGNGLVLW TVFRSSREKR RSADIFIASL AVADLTFVVT LPLWATYTYR DY DWPFGTF ACKLSSYLIF VNMYASVFCL TGLSFDRYLA IVRPVANARL RLRVSGAVAT AVLWVLAALL AMPVMVLRTT GDL ENTTKV QCYMDYSMVA TVSSEWAWEV GLGVSSTTVG FVVPFTIMLT CYFFIAQTIA GHFRKERIEG LRKRRRLLSI IVVL VVTFA LCWMPYHLVK TLYMLGSLLH WPCDFDLFLM NIFPYCTCIS YVNSCLNPFL YAFFDPRFRQ ACTSMLCCGQ SR |
-Macromolecule #5: scFv16
| Macromolecule | Name: scFv16 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 31.870629 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFADV QLVESGGGLV QPGGSRKLSC SASGFAFSSF GMHWVRQAPE KGLEWVAYI SSGSGTIYYA DTVKGRFTIS RDDPKNTLFL QMTSLRSEDT AMYYCVRSIY YYGSSPFDFW GQGTTLTVSS G GGGSGGGG ...String: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFADV QLVESGGGLV QPGGSRKLSC SASGFAFSSF GMHWVRQAPE KGLEWVAYI SSGSGTIYYA DTVKGRFTIS RDDPKNTLFL QMTSLRSEDT AMYYCVRSIY YYGSSPFDFW GQGTTLTVSS G GGGSGGGG SGGGGSDIVM TQATSSVPVT PGESVSISCR SSKSLLHSNG NTYLYWFLQR PGQSPQLLIY RMSNLASGVP DR FSGSGSG TAFTLTISRL EAEDVGVYYC MQHLEYPLTF GAGTKLELKA AAHHHHHHHH |
-Macromolecule #6: Apelin receptor early endogenous ligand
| Macromolecule | Name: Apelin receptor early endogenous ligand / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 3.980873 KDa |
| Sequence | String: QRPVNLTMRR KLRKHNCLQR RCMPLHSRVP FP |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.16 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 63197 |
|---|---|
| Initial angle assignment | Type: COMMON LINE |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller








































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)