[English] 日本語
 Yorodumi
Yorodumi- EMDB-31534: A complete three-dimensional structure of the Lon protease transl... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-31534 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | A complete three-dimensional structure of the Lon protease translocating a protein substrate (conformation 1) | |||||||||
 Map data Map data | A complete three-dimensional structure of the Lon protease translocating a protein substrate (conformation 1) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | AAA+ protease / Lon / complete three-dimensional structure / N-terminal domain / CYTOSOLIC PROTEIN / HYDROLASE-PROTEIN BINDING complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationendopeptidase La / ATP-dependent peptidase activity / protein quality control for misfolded or incompletely synthesized proteins / cellular response to heat / sequence-specific DNA binding / serine-type endopeptidase activity / ATP hydrolysis activity / ATP binding / metal ion binding / identical protein binding / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Meiothermus taiwanensis (bacteria) / Meiothermus taiwanensis (bacteria) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.4 Å | |||||||||
 Authors Authors | Li S / Hsieh K | |||||||||
| Funding support |  Taiwan, 1 items Taiwan, 1 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2021 Journal: Sci Adv / Year: 2021Title: Complete three-dimensional structures of the Lon protease translocating a protein substrate. Authors: Shanshan Li / Kan-Yen Hsieh / Chiao-I Kuo / Szu-Hui Lee / Grigore D Pintilie / Kaiming Zhang / Chung-I Chang /    Abstract: Lon is an evolutionarily conserved proteolytic machine carrying out a wide spectrum of biological activities by degrading misfolded damaged proteins and specific cellular substrates. Lon contains a ...Lon is an evolutionarily conserved proteolytic machine carrying out a wide spectrum of biological activities by degrading misfolded damaged proteins and specific cellular substrates. Lon contains a large N-terminal domain and forms a hexameric core of fused adenosine triphosphatase and protease domains. Here, we report two complete structures of Lon engaging a substrate, determined by cryo–electron microscopy to 2.4-angstrom resolution. These structures show a multilayered architecture featuring a tensegrity triangle complex, uniquely constructed by six long N-terminal helices. The interlocked helix triangle is assembled on the top of the hexameric core to spread a web of six globular substrate-binding domains. It serves as a multipurpose platform that controls the access of substrates to the AAA+ ring, provides a ruler-based mechanism for substrate selection, and acts as a pulley device to facilitate unfolding of the translocated substrate. This work provides a complete framework for understanding the structural mechanisms of Lon. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_31534.map.gz emd_31534.map.gz | 74.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-31534-v30.xml emd-31534-v30.xml emd-31534.xml emd-31534.xml | 15.3 KB 15.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_31534.png emd_31534.png | 88.5 KB | ||
| Filedesc metadata |  emd-31534.cif.gz emd-31534.cif.gz | 6.2 KB | ||
| Others |  emd_31534_additional_1.map.gz emd_31534_additional_1.map.gz | 136.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-31534 http://ftp.pdbj.org/pub/emdb/structures/EMD-31534 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31534 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31534 | HTTPS FTP |
-Related structure data
| Related structure data |  7fd4MC  7fd5C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_31534.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_31534.map.gz / Format: CCP4 / Size: 144.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | A complete three-dimensional structure of the Lon protease translocating a protein substrate (conformation 1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.82 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
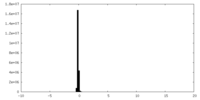
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: The N-terminal region of the Lon protease conformation 1
| File | emd_31534_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | The N-terminal region of the Lon protease conformation 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
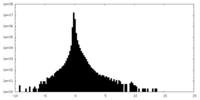
| Density Histograms |
- Sample components
Sample components
-Entire : A complete three-dimensional structure of the Lon protease transl...
| Entire | Name: A complete three-dimensional structure of the Lon protease translocating a protein substrate (conformation 1) |
|---|---|
| Components |
|
-Supramolecule #1: A complete three-dimensional structure of the Lon protease transl...
| Supramolecule | Name: A complete three-dimensional structure of the Lon protease translocating a protein substrate (conformation 1) type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Meiothermus taiwanensis (bacteria) Meiothermus taiwanensis (bacteria) |
| Molecular weight | Theoretical: 370 KDa |
-Supramolecule #2: Lon protease
| Supramolecule | Name: Lon protease / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: Alpha-S1-casein
| Supramolecule | Name: Alpha-S1-casein / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|
-Macromolecule #1: Lon protease
| Macromolecule | Name: Lon protease / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO / EC number: endopeptidase La |
|---|---|
| Source (natural) | Organism:  Meiothermus taiwanensis (bacteria) Meiothermus taiwanensis (bacteria) |
| Molecular weight | Theoretical: 88.554594 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MRLELPVIPL RNTVILPHTT TPVDVGRAKS KRAVEEAMGA DRLIFLVAQR DPEVDDPAPD DLYTWGVQAV VKQAMRLPDG TLQVMVEAR ARAQVTDYIP GPYLRARGEV FSEIFPIDEA VVRVLVEELK EAFEKYVANH KSLRLDRYQL EAVKGTSDPA M LADTIAYH ...String: MRLELPVIPL RNTVILPHTT TPVDVGRAKS KRAVEEAMGA DRLIFLVAQR DPEVDDPAPD DLYTWGVQAV VKQAMRLPDG TLQVMVEAR ARAQVTDYIP GPYLRARGEV FSEIFPIDEA VVRVLVEELK EAFEKYVANH KSLRLDRYQL EAVKGTSDPA M LADTIAYH ATWTVAEKQE ILELTDLEAR LKKVLGLLSR DLERFELDKR VAQRVKEQMD TNQREYYLRE QMKAIQKELG GE DGLSDLE ALRKKIEEVG MPEAVKTKAL KELDRLERMQ QGSPEATVAR TYLDWLTEVP WSKADPEVLD INHTRQVLDE DHY GLKDVK ERILEYLAVR QLTQGLDVRN KAPILVLVGP PGVGKTSLGR SIARSMNRKF HRISLGGVRD EAEIRGHRRT YIGA MPGKL IHAMKQVGVI NPVILLDEID KMSSDWRGDP ASAMLEVLDP EQNNTFTDHY LDVPYDLSKV FFITTANTLQ TIPRP LLDR MEVIEIPGYT NMEKQAIARQ YLWPKQVRES GMEGRIEVTD AAILRVISEY TREAGVRGLE RELGKIARKG AKFWLE GAW EGLRTIDASD IPTYLGIPRY RPDKAETEPQ VGTAQGLAWT PVGGTLLTIE VAAVPGSGKL SLTGQLGEVM KESAQAA LT YLRAHTQDYG LPEDFYNKVD LHVHVPDGAT PKDGPSAGIT MATAIASALS RRPARMDIAM TGEVSLRGKV MPIGGVKE K LLAAHQAGIH KIVLPKDNEA QLEELPKEVL EGLEIKLVED VGEVLEYLLL PEPTMPPVVQ PSDNRQQPGA GA UniProtKB: Lon protease |
-Macromolecule #2: Alpha-S1-casein
| Macromolecule | Name: Alpha-S1-casein / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 1.890321 KDa |
| Sequence | String: (UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK)(UNK)(UNK)(UNK) (UNK)(UNK)(UNK) (UNK)(UNK)(UNK) |
-Macromolecule #3: PHOSPHOTHIOPHOSPHORIC ACID-ADENYLATE ESTER
| Macromolecule | Name: PHOSPHOTHIOPHOSPHORIC ACID-ADENYLATE ESTER / type: ligand / ID: 3 / Number of copies: 4 / Formula: AGS |
|---|---|
| Molecular weight | Theoretical: 523.247 Da |
| Chemical component information |  ChemComp-AGS: |
-Macromolecule #4: N-[(1R)-1-(dihydroxyboranyl)-2-phenylethyl]-Nalpha-(pyrazin-2-ylc...
| Macromolecule | Name: N-[(1R)-1-(dihydroxyboranyl)-2-phenylethyl]-Nalpha-(pyrazin-2-ylcarbonyl)-L-phenylalaninamide type: ligand / ID: 4 / Number of copies: 6 / Formula: 4KZ |
|---|---|
| Molecular weight | Theoretical: 418.253 Da |
| Chemical component information |  ChemComp-4KZ: |
-Macromolecule #5: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 5 / Number of copies: 2 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 48.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 2.4 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 158553 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)