+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21267 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Chloroplast ATP synthase (R3, CF1) | |||||||||
 Map data Map data | R3, F1 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | CF1FO / ATP synthase / PHOTOSYNTHESIS / TRANSLOCASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationphotosynthetic electron transport in photosystem I / photosynthetic electron transport in photosystem II / chloroplast thylakoid membrane / proton motive force-driven ATP synthesis / proton motive force-driven mitochondrial ATP synthesis / H+-transporting two-sector ATPase / proton-transporting ATP synthase complex / proton-transporting ATP synthase activity, rotational mechanism / ADP binding / ATP hydrolysis activity ...photosynthetic electron transport in photosystem I / photosynthetic electron transport in photosystem II / chloroplast thylakoid membrane / proton motive force-driven ATP synthesis / proton motive force-driven mitochondrial ATP synthesis / H+-transporting two-sector ATPase / proton-transporting ATP synthase complex / proton-transporting ATP synthase activity, rotational mechanism / ADP binding / ATP hydrolysis activity / mitochondrion / ATP binding Similarity search - Function | |||||||||
| Biological species |  Spinacia oleracea (spinach) Spinacia oleracea (spinach) | |||||||||
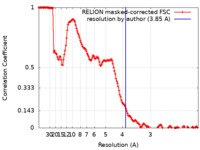
| Method | single particle reconstruction / cryo EM / Resolution: 3.85 Å | |||||||||
 Authors Authors | Yang J-H / Williams D | |||||||||
 Citation Citation |  Journal: Commun Biol / Year: 2020 Journal: Commun Biol / Year: 2020Title: Structural basis of redox modulation on chloroplast ATP synthase. Authors: Jay-How Yang / Dewight Williams / Eaazhisai Kandiah / Petra Fromme / Po-Lin Chiu /   Abstract: In higher plants, chloroplast ATP synthase has a unique redox switch on its γ subunit that modulates enzyme activity to limit ATP hydrolysis at night. To understand the molecular details of the ...In higher plants, chloroplast ATP synthase has a unique redox switch on its γ subunit that modulates enzyme activity to limit ATP hydrolysis at night. To understand the molecular details of the redox modulation, we used single-particle cryo-EM to determine the structures of spinach chloroplast ATP synthase in both reduced and oxidized states. The disulfide linkage of the oxidized γ subunit introduces a torsional constraint to stabilize the two β hairpin structures. Once reduced, free cysteines alleviate this constraint, resulting in a concerted motion of the enzyme complex and a smooth transition between rotary states to facilitate the ATP synthesis. We added an uncompetitive inhibitor, tentoxin, in the reduced sample to limit the flexibility of the enzyme and obtained high-resolution details. Our cryo-EM structures provide mechanistic insight into the redox modulation of the energy regulation activity of chloroplast ATP synthase. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21267.map.gz emd_21267.map.gz | 15.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21267-v30.xml emd-21267-v30.xml emd-21267.xml emd-21267.xml | 17.8 KB 17.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_21267_fsc.xml emd_21267_fsc.xml | 14.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_21267.png emd_21267.png | 62.6 KB | ||
| Filedesc metadata |  emd-21267.cif.gz emd-21267.cif.gz | 6.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21267 http://ftp.pdbj.org/pub/emdb/structures/EMD-21267 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21267 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21267 | HTTPS FTP |
-Related structure data
| Related structure data |  6vokMC  6vm1C  6vm4C  6vmbC  6vmdC  6vmgC  6vofC  6vogC  6vohC  6voiC  6vojC  6volC  6vomC  6vonC  6vooC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10475 (Title: Structural basis of redox modulation on chloroplast ATP synthase (reduced form) EMPIAR-10475 (Title: Structural basis of redox modulation on chloroplast ATP synthase (reduced form)Data size: 109.9 Data #1: Reduced state of the chloroplast ATP synthase [micrographs - single frame]) |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_21267.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21267.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | R3, F1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.053 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Chloroplast ATP synthase
| Entire | Name: Chloroplast ATP synthase |
|---|---|
| Components |
|
-Supramolecule #1: Chloroplast ATP synthase
| Supramolecule | Name: Chloroplast ATP synthase / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#5 / Details: Reduced rotary state 3 (CF1) |
|---|---|
| Source (natural) | Organism:  Spinacia oleracea (spinach) / Tissue: Leaves Spinacia oleracea (spinach) / Tissue: Leaves |
| Molecular weight | Theoretical: 594.35 KDa |
-Macromolecule #1: ATP synthase subunit alpha, chloroplastic
| Macromolecule | Name: ATP synthase subunit alpha, chloroplastic / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO / EC number: H+-transporting two-sector ATPase |
|---|---|
| Source (natural) | Organism:  Spinacia oleracea (spinach) Spinacia oleracea (spinach) |
| Molecular weight | Theoretical: 55.505199 KDa |
| Sequence | String: MATIRADEIS KIIRERIEGY NREVKVVNTG TVLQVGDGIA RIHGLDEVMA GELVEFEEGT IGIALNLESN NVGVVLMGDG LMIQEGSSV KATGRIAQIP VSEAYLGRVI NALAKPIDGR GEITASESRL IESPAPGIMS RRSVYEPLQT GLIAIDAMIP V GRGQRELI ...String: MATIRADEIS KIIRERIEGY NREVKVVNTG TVLQVGDGIA RIHGLDEVMA GELVEFEEGT IGIALNLESN NVGVVLMGDG LMIQEGSSV KATGRIAQIP VSEAYLGRVI NALAKPIDGR GEITASESRL IESPAPGIMS RRSVYEPLQT GLIAIDAMIP V GRGQRELI IGDRQTGKTA VATDTILNQQ GQNVICVYVA IGQKASSVAQ VVTNFQERGA MEYTIVVAET ADSPATLQYL AP YTGAALA EYFMYRERHT LIIYDDLSKQ AQAYRQMSLL LRRPPGREAY PGDVFYLHSR LLERAAKLSS LLGEGSMTAL PIV ETQAGD VSAYIPTNVI SITDGQIFLS ADLFNAGIRP AINVGISVSR VGSAAQIKAM KKVAGKLKLE LAQFAELEAF AQFA SDLDK ATQNQLARGQ RLRELLKQPQ SAPLTVEEQV MTIYTGTNGY LDSLELDQVR KYLVELRTYV KTNKPEFQEI ISSTK TFTE EAEALLKEAI QEQMERFLLQ EQA UniProtKB: ATP synthase subunit alpha, chloroplastic |
-Macromolecule #2: ATP synthase subunit beta, chloroplastic
| Macromolecule | Name: ATP synthase subunit beta, chloroplastic / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO / EC number: H+-transporting two-sector ATPase |
|---|---|
| Source (natural) | Organism:  Spinacia oleracea (spinach) Spinacia oleracea (spinach) |
| Molecular weight | Theoretical: 53.797367 KDa |
| Sequence | String: MRINPTTSDP GVSTLEKKNL GRIAQIIGPV LDVAFPPGKM PNIYNALIVK GRDTAGQPMN VTCEVQQLLG NNRVRAVAMS ATDGLTRGM EVIDTGAPLS VPVGGATLGR IFNVLGEPVD NLGPVDTRTT SPIHRSAPAF TQLDTKLSIF ETGIKVVDLL A PYRRGGKI ...String: MRINPTTSDP GVSTLEKKNL GRIAQIIGPV LDVAFPPGKM PNIYNALIVK GRDTAGQPMN VTCEVQQLLG NNRVRAVAMS ATDGLTRGM EVIDTGAPLS VPVGGATLGR IFNVLGEPVD NLGPVDTRTT SPIHRSAPAF TQLDTKLSIF ETGIKVVDLL A PYRRGGKI GLFGGAGVGK TVLIMELINN IAKAHGGVSV FGGVGERTRE GNDLYMEMKE SGVINEQNIA ESKVALVYGQ MN EPPGARM RVGLTALTMA EYFRDVNEQD VLLFIDNIFR FVQAGSEVSA LLGRMPSAVG YQPTLSTEMG SLQERITSTK EGS ITSIQA VYVPADDLTD PAPATTFAHL DATTVLSRGL AAKGIYPAVD PLDSTSTMLQ PRIVGEEHYE IAQRVKETLQ RYKE LQDII AILGLDELSE EDRLTVARAR KIERFLSQPF FVAEVFTGSP GKYVGLAETI RGFQLILSGE LDSLPEQAFY LVGNI DEAT AKAMNLEMES KLKK UniProtKB: ATP synthase subunit beta, chloroplastic |
-Macromolecule #3: ATP synthase delta chain, chloroplastic
| Macromolecule | Name: ATP synthase delta chain, chloroplastic / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Spinacia oleracea (spinach) Spinacia oleracea (spinach) |
| Molecular weight | Theoretical: 27.708582 KDa |
| Sequence | String: MAALQNPVAL QSRTTTAVAA LSTSSTTSTP KPFSLSFSSS TATFNPLRLK ILTASKLTAK PRGGALGTRM VDSTASRYAS ALADVADVT GTLEATNSDV EKLIRIFSEE PVYYFFANPV ISIDNKRSVL DEIITTSGLQ PHTANFINIL IDSERINLVK E ILNEFEDV ...String: MAALQNPVAL QSRTTTAVAA LSTSSTTSTP KPFSLSFSSS TATFNPLRLK ILTASKLTAK PRGGALGTRM VDSTASRYAS ALADVADVT GTLEATNSDV EKLIRIFSEE PVYYFFANPV ISIDNKRSVL DEIITTSGLQ PHTANFINIL IDSERINLVK E ILNEFEDV FNKITGTEVA VVTSVVKLEN DHLAQIAKGV QKITGAKNVR IKTVIDPSLV AGFTIRYGNE GSKLVDMSVK KQ LEEIAAQ LEMDDVTLAV UniProtKB: ATP synthase delta chain, chloroplastic |
-Macromolecule #4: ATP synthase epsilon chain, chloroplastic
| Macromolecule | Name: ATP synthase epsilon chain, chloroplastic / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Spinacia oleracea (spinach) Spinacia oleracea (spinach) |
| Molecular weight | Theoretical: 14.715707 KDa |
| Sequence | String: MTLNLCVLTP NRSIWNSEVK EIILSTNSGQ IGVLPNHAPT ATAVDIGILR IRLNDQWLTL ALMGGFARIG NNEITILVND AERGSDIDP QEAQQTLEIA EANLRKAEGK RQKIEANLAL RRARTRVEAS NTISS UniProtKB: ATP synthase epsilon chain, chloroplastic |
-Macromolecule #5: ATP synthase gamma chain, chloroplastic
| Macromolecule | Name: ATP synthase gamma chain, chloroplastic / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Spinacia oleracea (spinach) Spinacia oleracea (spinach) |
| Molecular weight | Theoretical: 40.119066 KDa |
| Sequence | String: MACSLSFSSS VSTFHLPTTT QSTQAPPNNA TTLPTTNPIQ CANLRELRDR IGSVKNTQKI TEAMKLVAAA KVRRAQEAVV NGRPFSETL VEVLYNMNEQ LQTEDVDVPL TKIRTVKKVA LMVVTGDRGL CGGFNNMLLK KAESRIAELK KLGVDYTIIS I GKKGNTYF ...String: MACSLSFSSS VSTFHLPTTT QSTQAPPNNA TTLPTTNPIQ CANLRELRDR IGSVKNTQKI TEAMKLVAAA KVRRAQEAVV NGRPFSETL VEVLYNMNEQ LQTEDVDVPL TKIRTVKKVA LMVVTGDRGL CGGFNNMLLK KAESRIAELK KLGVDYTIIS I GKKGNTYF IRRPEIPVDR YFDGTNLPTA KEAQAIADDV FSLFVSEEVD KVEMLYTKFV SLVKSDPVIH TLLPLSPKGE IC DINGKCV DAAEDELFRL TTKEGKLTVE RDMIKTETPA FSPILEFEQD PAQILDALLP LYLNSQILRA LQESLASELA ARM TAMSNA TDNANELKKT LSINYNRARQ AKITGEILEI VAGANACV UniProtKB: ATP synthase gamma chain, chloroplastic |
-Macromolecule #6: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 6 / Number of copies: 4 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Macromolecule #7: TENTOXIN
| Macromolecule | Name: TENTOXIN / type: ligand / ID: 7 / Number of copies: 1 / Formula: TTX |
|---|---|
| Molecular weight | Theoretical: 414.498 Da |
| Chemical component information |  ChemComp-TTX: |
-Macromolecule #8: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 8 / Number of copies: 2 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Grid | Model: C-flat-2/2 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE / Details: unspecified |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 43.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: -2.8000000000000003 µm / Nominal defocus min: -1.5 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)