+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-31019 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM structure of human Kv4.2-DPP6S-KChIP1 complex | |||||||||
 Map data Map data | map H | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | membrane protein | |||||||||
| Function / homology |  Function and homology information Function and homology informationKv4.3-KChIP1 channel complex / Kv4.2-KChIP2 channel complex / A-type (transient outward) potassium channel activity / Phase 1 - inactivation of fast Na+ channels / voltage-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / potassium ion export across plasma membrane / membrane repolarization / Voltage gated Potassium channels / regulation of potassium ion transmembrane transport / anchoring junction ...Kv4.3-KChIP1 channel complex / Kv4.2-KChIP2 channel complex / A-type (transient outward) potassium channel activity / Phase 1 - inactivation of fast Na+ channels / voltage-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / potassium ion export across plasma membrane / membrane repolarization / Voltage gated Potassium channels / regulation of potassium ion transmembrane transport / anchoring junction / postsynaptic specialization membrane / regulation of heart contraction / neuronal cell body membrane / plasma membrane raft / locomotor rhythm / action potential / voltage-gated potassium channel activity / potassium channel activity / neuronal action potential / potassium channel regulator activity / voltage-gated potassium channel complex / GABA-ergic synapse / muscle contraction / sensory perception of pain / potassium ion transmembrane transport / serine-type peptidase activity / protein localization to plasma membrane / protein homooligomerization / cytoplasmic side of plasma membrane / cellular response to hypoxia / chemical synaptic transmission / perikaryon / postsynaptic membrane / transmembrane transporter binding / dendritic spine / neuronal cell body / glutamatergic synapse / dendrite / calcium ion binding / synapse / proteolysis / membrane / metal ion binding / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
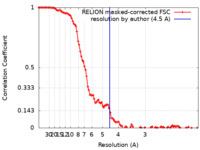
| Method | single particle reconstruction / cryo EM / Resolution: 4.5 Å | |||||||||
 Authors Authors | Kise Y / Nureki O | |||||||||
| Funding support |  Japan, 1 items Japan, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2021 Journal: Nature / Year: 2021Title: Structural basis of gating modulation of Kv4 channel complexes. Authors: Yoshiaki Kise / Go Kasuya / Hiroyuki H Okamoto / Daichi Yamanouchi / Kan Kobayashi / Tsukasa Kusakizako / Tomohiro Nishizawa / Koichi Nakajo / Osamu Nureki /  Abstract: Modulation of voltage-gated potassium (Kv) channels by auxiliary subunits is central to the physiological function of channels in the brain and heart. Native Kv4 tetrameric channels form ...Modulation of voltage-gated potassium (Kv) channels by auxiliary subunits is central to the physiological function of channels in the brain and heart. Native Kv4 tetrameric channels form macromolecular ternary complexes with two auxiliary β-subunits-intracellular Kv channel-interacting proteins (KChIPs) and transmembrane dipeptidyl peptidase-related proteins (DPPs)-to evoke rapidly activating and inactivating A-type currents, which prevent the backpropagation of action potentials. However, the modulatory mechanisms of Kv4 channel complexes remain largely unknown. Here we report cryo-electron microscopy structures of the Kv4.2-DPP6S-KChIP1 dodecamer complex, the Kv4.2-KChIP1 and Kv4.2-DPP6S octamer complexes, and Kv4.2 alone. The structure of the Kv4.2-KChIP1 complex reveals that the intracellular N terminus of Kv4.2 interacts with its C terminus that extends from the S6 gating helix of the neighbouring Kv4.2 subunit. KChIP1 captures both the N and the C terminus of Kv4.2. In consequence, KChIP1 would prevent N-type inactivation and stabilize the S6 conformation to modulate gating of the S6 helices within the tetramer. By contrast, unlike the reported auxiliary subunits of voltage-gated channel complexes, DPP6S interacts with the S1 and S2 helices of the Kv4.2 voltage-sensing domain, which suggests that DPP6S stabilizes the conformation of the S1-S2 helices. DPP6S may therefore accelerate the voltage-dependent movement of the S4 helices. KChIP1 and DPP6S do not directly interact with each other in the Kv4.2-KChIP1-DPP6S ternary complex. Thus, our data suggest that two distinct modes of modulation contribute in an additive manner to evoke A-type currents from the native Kv4 macromolecular complex. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_31019.map.gz emd_31019.map.gz | 17.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-31019-v30.xml emd-31019-v30.xml emd-31019.xml emd-31019.xml | 19.4 KB 19.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_31019_fsc.xml emd_31019_fsc.xml | 10.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_31019.png emd_31019.png | 37.6 KB | ||
| Masks |  emd_31019_msk_1.map emd_31019_msk_1.map | 101 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-31019.cif.gz emd-31019.cif.gz | 6.1 KB | ||
| Others |  emd_31019_half_map_1.map.gz emd_31019_half_map_1.map.gz emd_31019_half_map_2.map.gz emd_31019_half_map_2.map.gz | 77.7 MB 77.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-31019 http://ftp.pdbj.org/pub/emdb/structures/EMD-31019 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31019 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31019 | HTTPS FTP |
-Validation report
| Summary document |  emd_31019_validation.pdf.gz emd_31019_validation.pdf.gz | 773.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_31019_full_validation.pdf.gz emd_31019_full_validation.pdf.gz | 772.9 KB | Display | |
| Data in XML |  emd_31019_validation.xml.gz emd_31019_validation.xml.gz | 17.5 KB | Display | |
| Data in CIF |  emd_31019_validation.cif.gz emd_31019_validation.cif.gz | 22.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31019 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31019 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31019 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31019 | HTTPS FTP |
-Related structure data
| Related structure data |  7e8hMC  7e7zC  7e83C  7e84C  7e87C  7e89C  7e8bC  7e8eC  7e8gC  7f0jC  7f3fC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_31019.map.gz / Format: CCP4 / Size: 101 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_31019.map.gz / Format: CCP4 / Size: 101 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | map H | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.00268 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_31019_msk_1.map emd_31019_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_31019_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_31019_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : human Kv4.2-DPP6S-KChIP1 complex
| Entire | Name: human Kv4.2-DPP6S-KChIP1 complex |
|---|---|
| Components |
|
-Supramolecule #1: human Kv4.2-DPP6S-KChIP1 complex
| Supramolecule | Name: human Kv4.2-DPP6S-KChIP1 complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Dipeptidyl aminopeptidase-like protein 6
| Macromolecule | Name: Dipeptidyl aminopeptidase-like protein 6 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 86.277664 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: AKGIAIALLV ILVICSLIVT SVILLTPAED NSLSQKKKVT VEDLFSEDFK IHDPEAKWIS DTEFIYREQK GTVRLWNVET NTSTVLIEG KKIESLRAIR YEISPDREYA LFSYNVEPIY QHSYTGYYVL SKIPHGDPQS LDPPEVSNAK LQYAGWGPKG Q QLIFIFEN ...String: AKGIAIALLV ILVICSLIVT SVILLTPAED NSLSQKKKVT VEDLFSEDFK IHDPEAKWIS DTEFIYREQK GTVRLWNVET NTSTVLIEG KKIESLRAIR YEISPDREYA LFSYNVEPIY QHSYTGYYVL SKIPHGDPQS LDPPEVSNAK LQYAGWGPKG Q QLIFIFEN NIYYCAHVGK QAIRVVSTGK EGVIYNGLSD WLYEEEILKT HIAHWWSPDG TRLAYAAIND SRVPIMELPT YT GSIYPTV KPYHYPKAGS ENPSISLHVI GLNGPTHDLE MMPPDDPRMR EYYITMVKWA TSTKVAVTWL NRAQNVSILT LCD ATTGVC TKKHEDESEA WLHRQNEEPV FSKDGRKFFF IRAIPQGGRG KFYHITVSSS QPNSSNDNIQ SITSGDWDVT KILA YDEKG NKIYFLSTED LPRRRQLYSA NTVGNFNRQC LSCDLVENCT YFSASFSHSM DFFLLKCEGP GVPMVTVHNT TDKKK MFDL ETNEHVKKAI NDRQMPKVEY RDIEIDDYNL PMQILKPATF TDTTHYPLLL VVDGTPGSQS VAEKFEVSWE TVMVSS HGA VVVKCDGRGS GFQGTKLLHE VRRRLGLLEE KDQMEAVRTM LKEQYIDRTR VAVFGKDYGG YLSTYILPAK GENQGQT FT CGSALSPITD FKLYASAFSE RYLGLHGLDN RAYEMTKVAH RVSALEEQQF LIIHPTADEK IHFQHTAELI TQLIRGKA N YSLQIYPDES HYFTSSSLKQ HLYRSIINFF VECFRI UniProtKB: A-type potassium channel modulatory protein DPP6 |
-Macromolecule #2: Dipeptidyl aminopeptidase-like protein 6
| Macromolecule | Name: Dipeptidyl aminopeptidase-like protein 6 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 86.490898 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: AAAAKGIAIA LLVILVICSL IVTSVILLTP AEDNSLSQKK KVTVEDLFSE DFKIHDPEAK WISDTEFIYR EQKGTVRLWN VETNTSTVL IEGKKIESLR AIRYEISPDR EYALFSYNVE PIYQHSYTGY YVLSKIPHGD PQSLDPPEVS NAKLQYAGWG P KGQQLIFI ...String: AAAAKGIAIA LLVILVICSL IVTSVILLTP AEDNSLSQKK KVTVEDLFSE DFKIHDPEAK WISDTEFIYR EQKGTVRLWN VETNTSTVL IEGKKIESLR AIRYEISPDR EYALFSYNVE PIYQHSYTGY YVLSKIPHGD PQSLDPPEVS NAKLQYAGWG P KGQQLIFI FENNIYYCAH VGKQAIRVVS TGKEGVIYNG LSDWLYEEEI LKTHIAHWWS PDGTRLAYAA INDSRVPIME LP TYTGSIY PTVKPYHYPK AGSENPSISL HVIGLNGPTH DLEMMPPDDP RMREYYITMV KWATSTKVAV TWLNRAQNVS ILT LCDATT GVCTKKHEDE SEAWLHRQNE EPVFSKDGRK FFFIRAIPQG GRGKFYHITV SSSQPNSSND NIQSITSGDW DVTK ILAYD EKGNKIYFLS TEDLPRRRQL YSANTVGNFN RQCLSCDLVE NCTYFSASFS HSMDFFLLKC EGPGVPMVTV HNTTD KKKM FDLETNEHVK KAINDRQMPK VEYRDIEIDD YNLPMQILKP ATFTDTTHYP LLLVVDGTPG SQSVAEKFEV SWETVM VSS HGAVVVKCDG RGSGFQGTKL LHEVRRRLGL LEEKDQMEAV RTMLKEQYID RTRVAVFGKD YGGYLSTYIL PAKGENQ GQ TFTCGSALSP ITDFKLYASA FSERYLGLHG LDNRAYEMTK VAHRVSALEE QQFLIIHPTA DEKIHFQHTA ELITQLIR G KANYSLQIYP DESHYFTSSS LKQHLYRSII NFFVECFRI UniProtKB: A-type potassium channel modulatory protein DPP6 |
-Macromolecule #3: Kv channel-interacting protein 1
| Macromolecule | Name: Kv channel-interacting protein 1 / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 21.145826 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: EGLEQLEAQT NFTKRELQVL YRGFKNECPS GVVNEDTFKQ IYAQFFPHGD ASTYAHYLFN AFDTTQTGSV KFEDFVTALS ILLRGTVHE KLRWTFNLYD INKDGYINKE EMMDIVKAIY DMMGKYTYPV LKEDTPRQHV DVFFQKMDKN KDGIVTLDEF L ESCQEDDN IMRSLQLFQN VM UniProtKB: A-type potassium channel modulatory protein KCNIP1 |
-Macromolecule #4: Potassium voltage-gated channel subfamily D member 2
| Macromolecule | Name: Potassium voltage-gated channel subfamily D member 2 / type: protein_or_peptide / ID: 4 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 55.755738 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: AAGVAAWLPF ARAAAIGWMP VASGPMPAPP RQERKRTQDA LIVLNVSGTR FQTWQDTLER YPDTLLGSSE RDFFYHPETQ QYFFDRDPD IFRHILNFYR TGKLHYPRHE CISAYDEELA FFGLIPEIIG DCCYEEYKDR RRENAERLQD DADTDTAGES A LPTMTARQ ...String: AAGVAAWLPF ARAAAIGWMP VASGPMPAPP RQERKRTQDA LIVLNVSGTR FQTWQDTLER YPDTLLGSSE RDFFYHPETQ QYFFDRDPD IFRHILNFYR TGKLHYPRHE CISAYDEELA FFGLIPEIIG DCCYEEYKDR RRENAERLQD DADTDTAGES A LPTMTARQ RVWRAFENPH TSTMALVFYY VTGFFIAVSV IANVVETVPC GSSPGHIKEL PCGERYAVAF FCLDTACVMI FT VEYLLRL AAAPSRYRFV RSVMSIIDVV AILPYYIGLV MTDNEDVSGA FVTLRVFRVF RIFKFSRHSQ GLRILGYTLK SCA SELGFL LFSLTMAIII FATVMFYAEK GSSASKFTSI PAAFWYTIVT MTTLGYGDMV PKTIAGKIFG SICSLSGVLV IALP VPVIV SNFSRIYHQN QRADKRRAQK KARLARIRAA KSGSANAYMQ SKRSGLLSNQ LQSSEDEQAF VSKSGSSFET QHHHL LHCL EKTTNHEFVD UniProtKB: A-type voltage-gated potassium channel KCND2 |
-Macromolecule #5: Kv channel-interacting protein 1
| Macromolecule | Name: Kv channel-interacting protein 1 / type: protein_or_peptide / ID: 5 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 21.242941 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: PEGLEQLEAQ TNFTKRELQV LYRGFKNECP SGVVNEDTFK QIYAQFFPHG DASTYAHYLF NAFDTTQTGS VKFEDFVTAL SILLRGTVH EKLRWTFNLY DINKDGYINK EEMMDIVKAI YDMMGKYTYP VLKEDTPRQH VDVFFQKMDK NKDGIVTLDE F LESCQEDD NIMRSLQLFQ NVM UniProtKB: A-type potassium channel modulatory protein KCNIP1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 48.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)