+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30810 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structural basis for ligand binding modes of CTP synthase | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | substrate-bound / filament / LIGASE / DON | |||||||||
| Function / homology |  Function and homology information Function and homology informationInterconversion of nucleotide di- and triphosphates / larval lymph gland hemopoiesis / CTP synthase (glutamine hydrolysing) / CTP synthase activity / cytoophidium / 'de novo' CTP biosynthetic process / pyrimidine nucleobase biosynthetic process / CTP biosynthetic process / ATP binding / identical protein binding ...Interconversion of nucleotide di- and triphosphates / larval lymph gland hemopoiesis / CTP synthase (glutamine hydrolysing) / CTP synthase activity / cytoophidium / 'de novo' CTP biosynthetic process / pyrimidine nucleobase biosynthetic process / CTP biosynthetic process / ATP binding / identical protein binding / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
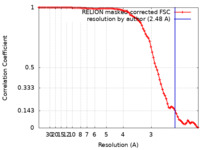
| Method | single particle reconstruction / cryo EM / Resolution: 2.48 Å | |||||||||
 Authors Authors | Liu JL / Zhou X | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2021 Journal: Proc Natl Acad Sci U S A / Year: 2021Title: Structural basis for ligand binding modes of CTP synthase. Authors: Xian Zhou / Chen-Jun Guo / Chia-Chun Chang / Jiale Zhong / Huan-Huan Hu / Guang-Ming Lu / Ji-Long Liu /   Abstract: Cytidine triphosphate synthase (CTPS), which comprises an ammonia ligase domain and a glutamine amidotransferase domain, catalyzes the final step of de novo CTP biosynthesis. The activity of CTPS is ...Cytidine triphosphate synthase (CTPS), which comprises an ammonia ligase domain and a glutamine amidotransferase domain, catalyzes the final step of de novo CTP biosynthesis. The activity of CTPS is regulated by the binding of four nucleotides and glutamine. While glutamine serves as an ammonia donor for the ATP-dependent conversion of UTP to CTP, the fourth nucleotide GTP acts as an allosteric activator. Models have been proposed to explain the mechanisms of action at the active site of the ammonia ligase domain and the conformational changes derived by GTP binding. However, actual GTP/ATP/UTP binding modes and relevant conformational changes have not been revealed fully. Here, we report the discovery of binding modes of four nucleotides and a glutamine analog 6-diazo-5-oxo-L-norleucine in CTPS by cryo-electron microscopy with near-atomic resolution. Interactions between GTP and surrounding residues indicate that GTP acts to coordinate reactions at both domains by directly blocking ammonia leakage and stabilizing the ammonia tunnel. Additionally, we observe the ATP-dependent UTP phosphorylation intermediate and determine interacting residues at the ammonia ligase. A noncanonical CTP binding at the ATP binding site suggests another layer of feedback inhibition. Our findings not only delineate the structure of CTPS in the presence of all substrates but also complete our understanding of the underlying mechanisms of the allosteric regulation and CTP synthesis. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30810.map.gz emd_30810.map.gz | 37.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30810-v30.xml emd-30810-v30.xml emd-30810.xml emd-30810.xml | 16 KB 16 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_30810_fsc.xml emd_30810_fsc.xml | 8.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_30810.png emd_30810.png | 65.9 KB | ||
| Masks |  emd_30810_msk_1.map emd_30810_msk_1.map | 59.6 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-30810.cif.gz emd-30810.cif.gz | 5.8 KB | ||
| Others |  emd_30810_half_map_1.map.gz emd_30810_half_map_1.map.gz emd_30810_half_map_2.map.gz emd_30810_half_map_2.map.gz | 44.9 MB 44.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30810 http://ftp.pdbj.org/pub/emdb/structures/EMD-30810 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30810 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30810 | HTTPS FTP |
-Related structure data
| Related structure data |  7dptMC  7dpwC  7wizC  7wj4C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30810.map.gz / Format: CCP4 / Size: 59.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30810.map.gz / Format: CCP4 / Size: 59.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_30810_msk_1.map emd_30810_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_30810_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_30810_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Drosophila substrate-bound CTP synthase
| Entire | Name: Drosophila substrate-bound CTP synthase |
|---|---|
| Components |
|
-Supramolecule #1: Drosophila substrate-bound CTP synthase
| Supramolecule | Name: Drosophila substrate-bound CTP synthase / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: CTP synthase
| Macromolecule | Name: CTP synthase / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO / EC number: CTP synthase (glutamine hydrolysing) |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 69.539453 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKYILVTGGV ISGVGKGVIA SSFGTLLKSC GLDVTSIKID PYINIDAGTF SPYEHGEVYV LDDGAEVDLD LGNYERFLDV TLHRDNNIT TGKIYKLVIE KERTGEYLGK TVQVVPHITD AIQEWVERVA QTPVQGSSKP QVCIVELGGT IGDIEGMPFV E AFRQFQFR ...String: MKYILVTGGV ISGVGKGVIA SSFGTLLKSC GLDVTSIKID PYINIDAGTF SPYEHGEVYV LDDGAEVDLD LGNYERFLDV TLHRDNNIT TGKIYKLVIE KERTGEYLGK TVQVVPHITD AIQEWVERVA QTPVQGSSKP QVCIVELGGT IGDIEGMPFV E AFRQFQFR VKRENFCLAH VSLVPLPKAT GEPKTKPTQS SVRELRGCGL SPDLIVCRSE KPIGLEVKEK ISNFCHVGPD QV ICIHDLN SIYHVPLLME QNGVIEYLNE RLQLNIDMSK RTKCLQQWRD LARRTETVRR EVCIAVVGKY TKFTDSYASV VKA LQHAAL AVNRKLELVF IESCLLEEET LHSEPSKYHK EWQKLCDSHG ILVPGGFGSR GMEGKIRACQ WARENQKPLL GICL GLQAA VIEFARNKLG LKDANTTEID PNTANALVID MPEHHTGQLG GTMRLGKRIT VFSDGPSVIR QLYGNPKSVQ ERHRH RYEV NPKYVHLLEE QGMRFVGTDV DKTRMEIIEL SGHPYFVATQ YHPEYLSRPL KPSPPFLGLI LASVDRLNQY IQRGCR LSP RQLSDASSDE EDSVVGLAGA TKSLSSLKIP ITPTNGISKS CNGSISTSDS EGACGGVDPT NGHK UniProtKB: CTP synthase |
-Macromolecule #2: GUANOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: GUANOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 2 / Number of copies: 4 / Formula: GTP |
|---|---|
| Molecular weight | Theoretical: 523.18 Da |
| Chemical component information |  ChemComp-GTP: |
-Macromolecule #3: 6-DIAZENYL-5-OXO-L-NORLEUCINE
| Macromolecule | Name: 6-DIAZENYL-5-OXO-L-NORLEUCINE / type: ligand / ID: 3 / Number of copies: 4 / Formula: DON |
|---|---|
| Molecular weight | Theoretical: 173.17 Da |
| Chemical component information |  ChemComp-DON: |
-Macromolecule #4: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 4 / Number of copies: 4 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #5: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 5 / Number of copies: 12 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #6: [[(2~{R},3~{S},4~{R},5~{R})-3,4-bis(oxidanyl)-5-(2-oxidanyl-4-pho...
| Macromolecule | Name: [[(2~{R},3~{S},4~{R},5~{R})-3,4-bis(oxidanyl)-5-(2-oxidanyl-4-phosphonooxy-pyrimidin-1-yl)oxolan-2-yl]methoxy-oxidanyl-phosphoryl] phosphono hydrogen phosphate type: ligand / ID: 6 / Number of copies: 4 / Formula: 5ZL |
|---|---|
| Molecular weight | Theoretical: 565.129 Da |
| Chemical component information | 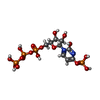 ChemComp-5ZL: |
-Macromolecule #7: water
| Macromolecule | Name: water / type: ligand / ID: 7 / Number of copies: 96 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: DARK FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)