[English] 日本語
 Yorodumi
Yorodumi- EMDB-28186: Structure of HIV-1 capsid declination in complex with CPSF6-FG peptide -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of HIV-1 capsid declination in complex with CPSF6-FG peptide | |||||||||
 Map data Map data | HIV-1 capsid declination in complex with CPSF6-FG peptide | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | HIV-1 / capsid / declination / pentamer / hexamer / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationexon-exon junction complex binding / positive regulation of RNA export from nucleus / mRNA cleavage factor complex / interchromatin granule / co-transcriptional mRNA 3'-end processing, cleavage and polyadenylation pathway / perichromatin fibrils / Processing of Intronless Pre-mRNAs / mRNA alternative polyadenylation / mRNA cleavage and polyadenylation specificity factor complex / mRNA 3'-end processing ...exon-exon junction complex binding / positive regulation of RNA export from nucleus / mRNA cleavage factor complex / interchromatin granule / co-transcriptional mRNA 3'-end processing, cleavage and polyadenylation pathway / perichromatin fibrils / Processing of Intronless Pre-mRNAs / mRNA alternative polyadenylation / mRNA cleavage and polyadenylation specificity factor complex / mRNA 3'-end processing / Signaling by cytosolic FGFR1 fusion mutants / mRNA 3'-end processing / paraspeckles / RNA Polymerase II Transcription Termination / protein heterotetramerization / viral budding via host ESCRT complex / ribosomal large subunit binding / Processing of Capped Intron-Containing Pre-mRNA / Signaling by FGFR1 in disease / protein tetramerization / ISG15 antiviral mechanism / host multivesicular body / mRNA processing / viral nucleocapsid / nuclear speck / ribonucleoprotein complex / viral translational frameshifting / mRNA binding / host cell nucleus / host cell plasma membrane / virion membrane / structural molecule activity / RNA binding / zinc ion binding / nucleoplasm / nucleus / membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |   Human immunodeficiency virus 1 / Human immunodeficiency virus 1 /  Homo sapiens (human) Homo sapiens (human) | |||||||||
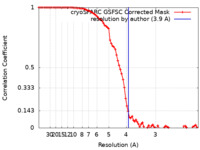
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||
 Authors Authors | Pornillos O / Ganser-Pornillos BK / Schirra RT / dos Santos NFB | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2023 Journal: Nat Struct Mol Biol / Year: 2023Title: A molecular switch modulates assembly and host factor binding of the HIV-1 capsid. Authors: Randall T Schirra / Nayara F B Dos Santos / Kaneil K Zadrozny / Iga Kucharska / Barbie K Ganser-Pornillos / Owen Pornillos /   Abstract: The HIV-1 capsid is a fullerene cone made of quasi-equivalent hexamers and pentamers of the viral CA protein. Typically, quasi-equivalent assembly of viral capsid subunits is controlled by a ...The HIV-1 capsid is a fullerene cone made of quasi-equivalent hexamers and pentamers of the viral CA protein. Typically, quasi-equivalent assembly of viral capsid subunits is controlled by a molecular switch. Here, we identify a Thr-Val-Gly-Gly motif that modulates CA hexamer/pentamer switching by folding into a 3 helix in the pentamer and random coil in the hexamer. Manipulating the coil/helix configuration of the motif allowed us to control pentamer and hexamer formation in a predictable manner, thus proving its function as a molecular switch. Importantly, the switch also remodels the common binding site for host factors that are critical for viral replication and the new ultra-potent HIV-1 inhibitor lenacapavir. This study reveals that a critical assembly element also modulates the post-assembly and viral replication functions of the HIV-1 capsid and provides new insights on capsid function and inhibition. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28186.map.gz emd_28186.map.gz | 57.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28186-v30.xml emd-28186-v30.xml emd-28186.xml emd-28186.xml | 17.2 KB 17.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_28186_fsc.xml emd_28186_fsc.xml | 8.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_28186.png emd_28186.png | 181 KB | ||
| Filedesc metadata |  emd-28186.cif.gz emd-28186.cif.gz | 5.6 KB | ||
| Others |  emd_28186_half_map_1.map.gz emd_28186_half_map_1.map.gz emd_28186_half_map_2.map.gz emd_28186_half_map_2.map.gz | 56.3 MB 56.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28186 http://ftp.pdbj.org/pub/emdb/structures/EMD-28186 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28186 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28186 | HTTPS FTP |
-Related structure data
| Related structure data |  8ejlMC  7urnC  7urtC  8eepC  8eetC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28186.map.gz / Format: CCP4 / Size: 61 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28186.map.gz / Format: CCP4 / Size: 61 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | HIV-1 capsid declination in complex with CPSF6-FG peptide | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_28186_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
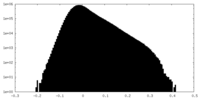
| Projections & Slices |
| ||||||||||||
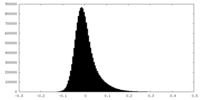
| Density Histograms |
-Half map: #2
| File | emd_28186_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
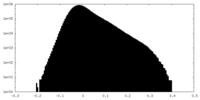
| Projections & Slices |
| ||||||||||||
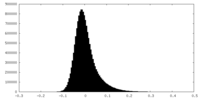
| Density Histograms |
- Sample components
Sample components
-Entire : Capsid-like particles of in vitro assembled HIV-1 CA protein in c...
| Entire | Name: Capsid-like particles of in vitro assembled HIV-1 CA protein in complex with CPSF6-FG peptide |
|---|---|
| Components |
|
-Supramolecule #1: Capsid-like particles of in vitro assembled HIV-1 CA protein in c...
| Supramolecule | Name: Capsid-like particles of in vitro assembled HIV-1 CA protein in complex with CPSF6-FG peptide type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: Capsids (0.27 mM CA) were incubated with peptide (3 mM) for 1 hour in ice |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
-Macromolecule #1: HIV-1 capsid protein
| Macromolecule | Name: HIV-1 capsid protein / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 / Strain: NL4-3 Human immunodeficiency virus 1 / Strain: NL4-3 |
| Molecular weight | Theoretical: 25.630426 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: PIVQNLQGQM VHQAISPRTL NAWVKVVEEK AFSPEVIPMF SALSEGATPQ DLNTMLNTVG GHQAAMQMLK ETINEEAAEW DRLHPVHAG PIAPGQMREP RGSDIAGTTS TLQEQIGWMT HNPPIPVGEI YKRWIILGLN KIVRMYSPTS ILDIRQGPKE P FRDYVDRF ...String: PIVQNLQGQM VHQAISPRTL NAWVKVVEEK AFSPEVIPMF SALSEGATPQ DLNTMLNTVG GHQAAMQMLK ETINEEAAEW DRLHPVHAG PIAPGQMREP RGSDIAGTTS TLQEQIGWMT HNPPIPVGEI YKRWIILGLN KIVRMYSPTS ILDIRQGPKE P FRDYVDRF YKTLRAEQAS QEVKNWMTET LLVQNANPDC KTILKALGPG ATLEEMMTAC QGVGGPGHKA RVL UniProtKB: Gag polyprotein |
-Macromolecule #2: Cleavage and polyadenylation specificity factor subunit 6
| Macromolecule | Name: Cleavage and polyadenylation specificity factor subunit 6 type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 1.708952 KDa |
| Sequence | String: GTPVLFPGQP FGQPPLG UniProtKB: Cleavage and polyadenylation specificity factor subunit 6 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 6 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Instrument: HOMEMADE PLUNGER / Details: Manual plunge-freezing. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.5 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: OTHER | ||||||||||||
| Output model |  PDB-8ejl: |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)