+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-2540 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | cryo-electron microscopy of microtubule-bound human kinesin-5 motor domain in the ADP state (gold cluster in the N-terminus A9C). | |||||||||
 マップデータ マップデータ | Reconstruction of microtubule-bound human kinesin-5 motor domain in presence of ADP and with a glod cluster covalently attached to the residue A9C | |||||||||
 試料 試料 |
| |||||||||
 キーワード キーワード | cryo-electron microscopy / kinesins / microtubules / mitosis / mechanochemistry | |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報spindle elongation / regulation of mitotic centrosome separation / Kinesins / plus-end-directed microtubule motor activity / mitotic centrosome separation / positive regulation of axon guidance / COPI-dependent Golgi-to-ER retrograde traffic / microtubule motor activity / kinesin complex / spindle organization ...spindle elongation / regulation of mitotic centrosome separation / Kinesins / plus-end-directed microtubule motor activity / mitotic centrosome separation / positive regulation of axon guidance / COPI-dependent Golgi-to-ER retrograde traffic / microtubule motor activity / kinesin complex / spindle organization / microtubule-based movement / microtubule-based process / mitotic spindle assembly / MHC class II antigen presentation / mitotic spindle organization / structural constituent of cytoskeleton / mitotic spindle / microtubule cytoskeleton organization / spindle pole / spindle / microtubule cytoskeleton / mitotic cell cycle / nervous system development / 加水分解酵素; 酸無水物に作用; GTPに作用・細胞または細胞小器官の運動に関与 / microtubule binding / microtubule / hydrolase activity / protein heterodimerization activity / cell division / GTPase activity / GTP binding / protein kinase binding / protein-containing complex / ATP binding / membrane / nucleus / metal ion binding / cytosol / cytoplasm 類似検索 - 分子機能 | |||||||||
| 生物種 |   Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 18.0 Å | |||||||||
 データ登録者 データ登録者 | GOULET A / MAJOR J / JUN Y / GROSS SP / ROSENFELD SR / MOORES CA | |||||||||
 引用 引用 |  ジャーナル: Proc Natl Acad Sci U S A / 年: 2014 ジャーナル: Proc Natl Acad Sci U S A / 年: 2014タイトル: Comprehensive structural model of the mechanochemical cycle of a mitotic motor highlights molecular adaptations in the kinesin family. 著者: Adeline Goulet / Jennifer Major / Yonggun Jun / Steven P Gross / Steven S Rosenfeld / Carolyn A Moores /  要旨: Kinesins are responsible for a wide variety of microtubule-based, ATP-dependent functions. Their motor domain drives these activities, but the molecular adaptations that specify these diverse and ...Kinesins are responsible for a wide variety of microtubule-based, ATP-dependent functions. Their motor domain drives these activities, but the molecular adaptations that specify these diverse and essential cellular activities are poorly understood. It has been assumed that the first identified kinesin--the transport motor kinesin-1--is the mechanistic paradigm for the entire superfamily, but accumulating evidence suggests otherwise. To address the deficits in our understanding of the molecular basis of functional divergence within the kinesin superfamily, we studied kinesin-5s, which are essential mitotic motors whose inhibition blocks cell division. Using cryo-electron microscopy and determination of structure at subnanometer resolution, we have visualized conformations of microtubule-bound human kinesin-5 motor domain at successive steps in its ATPase cycle. After ATP hydrolysis, nucleotide-dependent conformational changes in the active site are allosterically propagated into rotations of the motor domain and uncurling of the drug-binding loop L5. In addition, the mechanical neck-linker element that is crucial for motor stepping undergoes discrete, ordered displacements. We also observed large reorientations of the motor N terminus that indicate its importance for kinesin-5 function through control of neck-linker conformation. A kinesin-5 mutant lacking this N terminus is enzymatically active, and ATP-dependent neck-linker movement and motility are defective, although not ablated. All these aspects of kinesin-5 mechanochemistry are distinct from kinesin-1. Our findings directly demonstrate the regulatory role of the kinesin-5 N terminus in collaboration with the motor's structured neck-linker and highlight the multiple adaptations within kinesin motor domains that tune their mechanochemistries according to distinct functional requirements. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_2540.map.gz emd_2540.map.gz | 459.7 KB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-2540-v30.xml emd-2540-v30.xml emd-2540.xml emd-2540.xml | 11 KB 11 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_2540.jpg emd_2540.jpg | 140.2 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-2540 http://ftp.pdbj.org/pub/emdb/structures/EMD-2540 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2540 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-2540 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_2540_validation.pdf.gz emd_2540_validation.pdf.gz | 223.4 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_2540_full_validation.pdf.gz emd_2540_full_validation.pdf.gz | 222.6 KB | 表示 | |
| XML形式データ |  emd_2540_validation.xml.gz emd_2540_validation.xml.gz | 4.8 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2540 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2540 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2540 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-2540 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_2540.map.gz / 形式: CCP4 / 大きさ: 478.5 KB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_2540.map.gz / 形式: CCP4 / 大きさ: 478.5 KB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | Reconstruction of microtubule-bound human kinesin-5 motor domain in presence of ADP and with a glod cluster covalently attached to the residue A9C | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 2.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
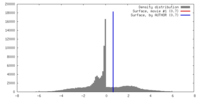
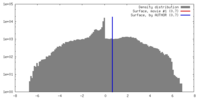
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : 13-protofilament microtubule-bound human kinesin-5 motor domain w...
| 全体 | 名称: 13-protofilament microtubule-bound human kinesin-5 motor domain with ADP bound. A gold cluster is attached to the N-terminus (A9C). |
|---|---|
| 要素 |
|
-超分子 #1000: 13-protofilament microtubule-bound human kinesin-5 motor domain w...
| 超分子 | 名称: 13-protofilament microtubule-bound human kinesin-5 motor domain with ADP bound. A gold cluster is attached to the N-terminus (A9C). タイプ: sample / ID: 1000 集合状態: 13-protofilament microtubule with one kinesin-5 motor domain bound every tubulin heterodimers Number unique components: 3 |
|---|
-分子 #1: alpha tubulin
| 分子 | 名称: alpha tubulin / タイプ: protein_or_peptide / ID: 1 / Name.synonym: TUBULIN ALPHA-1D CHAIN / 組換発現: No |
|---|---|
| 由来(天然) | 生物種:  |
| 配列 | UniProtKB: Tubulin alpha-1D chain / InterPro: Alpha tubulin |
-分子 #2: beta tubulin
| 分子 | 名称: beta tubulin / タイプ: protein_or_peptide / ID: 2 / Name.synonym: TUBULIN BETA-2B CHAIN / 組換発現: No |
|---|---|
| 由来(天然) | 生物種:  |
| 配列 | UniProtKB: Tubulin beta-2B chain / InterPro: Beta tubulin, autoregulation binding site |
-分子 #3: Kinesin-5 motor domain
| 分子 | 名称: Kinesin-5 motor domain / タイプ: protein_or_peptide / ID: 3 / Name.synonym: KINESIN-LIKE PROTEIN KIF11 詳細: cys-lite mutant containing the substitution A9C, undecagold cluster was attached to the specific cysteine residue in the N-terminus (A9C) 組換発現: Yes |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) / 別称: Human Homo sapiens (ヒト) / 別称: Human |
| 組換発現 | 生物種:  |
| 配列 | UniProtKB: Kinesin-like protein KIF11 / InterPro: Kinesin motor domain, conserved site |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 6.8 詳細: 20 mM PIPES, 5 mM MgCl2, 1 mM EGTA, 10 mM ADP, 2% glycerol |
|---|---|
| グリッド | 詳細: 400 mesh holey carbon grids |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / 装置: FEI VITROBOT MARK I / 手法: chamber at 24 degrees C, blot 3.5 sec |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TECNAI F20 |
|---|---|
| 温度 | 平均: 90 K |
| アライメント法 | Legacy - 非点収差: Objective lens astigmatism was corrected at 150,000 times magnification |
| 日付 | 2013年3月20日 |
| 撮影 | カテゴリ: CCD フィルム・検出器のモデル: GATAN ULTRASCAN 4000 (4k x 4k) 実像数: 23 / 平均電子線量: 18 e/Å2 |
| 電子線 | 加速電圧: 200 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 倍率(補正後): 68000 / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.0 mm / 最大 デフォーカス(公称値): 2.6 µm / 最小 デフォーカス(公称値): 1.1 µm |
| 試料ステージ | 試料ホルダーモデル: GATAN LIQUID NITROGEN |
| 実験機器 |  モデル: Tecnai F20 / 画像提供: FEI Company |
- 画像解析
画像解析
| 詳細 | The particles were selected along individual microtubules. |
|---|---|
| CTF補正 | 詳細: FREALIGN |
| 最終 再構成 | 想定した対称性 - 点群: C1 (非対称) / 解像度のタイプ: BY AUTHOR / 解像度: 18.0 Å / 解像度の算出法: OTHER / ソフトウェア - 名称: SPIDER, FREALIGN 詳細: Approximately 14,000 asymmetric units were averaged in the final reconstruction. 使用した粒子像数: 1080 |
 ムービー
ムービー コントローラー
コントローラー





































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)