[English] 日本語
 Yorodumi
Yorodumi- EMDB-24839: Human KATP channel in open conformation, focused on Kir (C166S G3... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24839 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human KATP channel in open conformation, focused on Kir (C166S G334D double mutant) and SUR TMD0 | |||||||||
 Map data Map data | map focus-refined on Kir and SUR TMD0 after sharpening (B-factor: 77.1) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ion channel / KATP / ATP-sensitive potassium channel / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationresponse to resveratrol / ATP-activated inward rectifier potassium channel activity / inward rectifying potassium channel / ventricular cardiac muscle tissue development / cell body fiber / CAMKK-AMPK signaling cascade / ATPase-coupled monoatomic cation transmembrane transporter activity / ankyrin binding / response to ATP / potassium ion import across plasma membrane ...response to resveratrol / ATP-activated inward rectifier potassium channel activity / inward rectifying potassium channel / ventricular cardiac muscle tissue development / cell body fiber / CAMKK-AMPK signaling cascade / ATPase-coupled monoatomic cation transmembrane transporter activity / ankyrin binding / response to ATP / potassium ion import across plasma membrane / response to testosterone / action potential / axolemma / intercalated disc / cellular response to nutrient levels / negative regulation of insulin secretion / heat shock protein binding / T-tubule / acrosomal vesicle / response to ischemia / determination of adult lifespan / positive regulation of protein localization to plasma membrane / cellular response to glucose stimulus / cellular response to nicotine / nuclear envelope / cellular response to tumor necrosis factor / response to estradiol / presynaptic membrane / transmembrane transporter binding / response to hypoxia / endosome / response to xenobiotic stimulus / neuronal cell body / apoptotic process / glutamatergic synapse / ATP binding Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
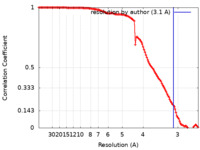
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Zhao C / MacKinnon R | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2021 Journal: Proc Natl Acad Sci U S A / Year: 2021Title: Molecular structure of an open human K channel. Authors: Chen Zhao / Roderick MacKinnon /  Abstract: K channels are metabolic sensors that translate intracellular ATP/ADP balance into membrane excitability. The molecular composition of K includes an inward-rectifier potassium channel (Kir) and an ...K channels are metabolic sensors that translate intracellular ATP/ADP balance into membrane excitability. The molecular composition of K includes an inward-rectifier potassium channel (Kir) and an ABC transporter-like sulfonylurea receptor (SUR). Although structures of K have been determined in many conformations, in all cases, the pore in Kir is closed. Here, we describe human pancreatic K (hK) structures with an open pore at 3.1- to 4.0-Å resolution using single-particle cryo-electron microscopy (cryo-EM). Pore opening is associated with coordinated structural changes within the ATP-binding site and the channel gate in Kir. Conformational changes in SUR are also observed, resulting in an area reduction of contact surfaces between SUR and Kir. We also observe that pancreatic hK exhibits the unique (among inward-rectifier channels) property of PIP-independent opening, which appears to be correlated with a docked cytoplasmic domain in the absence of PIP. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24839.map.gz emd_24839.map.gz | 173.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24839-v30.xml emd-24839-v30.xml emd-24839.xml emd-24839.xml | 13.3 KB 13.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_24839_fsc.xml emd_24839_fsc.xml | 12.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_24839.png emd_24839.png | 187.3 KB | ||
| Filedesc metadata |  emd-24839.cif.gz emd-24839.cif.gz | 5.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24839 http://ftp.pdbj.org/pub/emdb/structures/EMD-24839 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24839 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24839 | HTTPS FTP |
-Validation report
| Summary document |  emd_24839_validation.pdf.gz emd_24839_validation.pdf.gz | 516.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_24839_full_validation.pdf.gz emd_24839_full_validation.pdf.gz | 516.3 KB | Display | |
| Data in XML |  emd_24839_validation.xml.gz emd_24839_validation.xml.gz | 13 KB | Display | |
| Data in CIF |  emd_24839_validation.cif.gz emd_24839_validation.cif.gz | 17.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24839 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24839 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24839 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24839 | HTTPS FTP |
-Related structure data
| Related structure data |  7s5tMC  7s5vC  7s5xC  7s5yC  7s5zC  7s60C  7s61C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_24839.map.gz / Format: CCP4 / Size: 184 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24839.map.gz / Format: CCP4 / Size: 184 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | map focus-refined on Kir and SUR TMD0 after sharpening (B-factor: 77.1) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.3 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Human KATP channel composed of Kir6.2 (C166S G334D) and SUR1
| Entire | Name: Human KATP channel composed of Kir6.2 (C166S G334D) and SUR1 |
|---|---|
| Components |
|
-Supramolecule #1: Human KATP channel composed of Kir6.2 (C166S G334D) and SUR1
| Supramolecule | Name: Human KATP channel composed of Kir6.2 (C166S G334D) and SUR1 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 881.87 kDa/nm |
-Macromolecule #1: ATP-sensitive inward rectifier potassium channel 11
| Macromolecule | Name: ATP-sensitive inward rectifier potassium channel 11 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 43.622746 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MLSRKGIIPE EYVLTRLAED PAKPRYRARQ RRARFVSKKG NCNVAHKNIR EQGRFLQDVF TTLVDLKWPH TLLIFTMSFL CSWLLFAMA WWLIAFAHGD LAPSEGTAEP CVTSIHSFSS AFLFSIEVQV TIGFGGRMVT EECPLAILIL IVQNIVGLMI N AIMLGSIF ...String: MLSRKGIIPE EYVLTRLAED PAKPRYRARQ RRARFVSKKG NCNVAHKNIR EQGRFLQDVF TTLVDLKWPH TLLIFTMSFL CSWLLFAMA WWLIAFAHGD LAPSEGTAEP CVTSIHSFSS AFLFSIEVQV TIGFGGRMVT EECPLAILIL IVQNIVGLMI N AIMLGSIF MKTAQAHRRA ETLIFSKHAV IALRHGRLCF MLRVGDLRKS MIISATIHMQ VVRKTTSPEG EVVPLHQVDI PM ENGVGGN SIFLVAPLII YHVIDANSPL YDLAPSDLHH HQDLEIIVIL EGVVETTGIT TQARTSYLAD EILWGQRFVP IVA EEDGRY SVDYSKFDNT VKVPTPLCTA RQLDEDHSLL EALTLASARG PLRKRSVPMA KAKPKFSISP DSLS UniProtKB: ATP-sensitive inward rectifier potassium channel 11 |
-Macromolecule #2: POTASSIUM ION
| Macromolecule | Name: POTASSIUM ION / type: ligand / ID: 2 / Number of copies: 2 / Formula: K |
|---|---|
| Molecular weight | Theoretical: 39.098 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 6.83 mg/mL |
|---|---|
| Buffer | pH: 8.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 289 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 57.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)