[English] 日本語
 Yorodumi
Yorodumi- EMDB-22490: Structure of human TRPA1 in complex with antagonist compound 21 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22490 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of human TRPA1 in complex with antagonist compound 21 | |||||||||
 Map data Map data | Output from density modification in Phenix | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | TRPA1 / channel / agonist / MEMBRANE PROTEIN / MEMBRANE PROTEIN-Antagonist complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of blood circulation / positive regulation of monoatomic anion transport / cellular response to food / temperature-gated cation channel activity / regulation of neuronal action potential / cellular response to carbon dioxide / osmolarity-sensing monoatomic cation channel activity / stereocilium bundle / detection of chemical stimulus involved in sensory perception of pain / urinary bladder smooth muscle contraction ...regulation of blood circulation / positive regulation of monoatomic anion transport / cellular response to food / temperature-gated cation channel activity / regulation of neuronal action potential / cellular response to carbon dioxide / osmolarity-sensing monoatomic cation channel activity / stereocilium bundle / detection of chemical stimulus involved in sensory perception of pain / urinary bladder smooth muscle contraction / thermoception / cellular response to toxic substance / cellular response to caffeine / response to pain / TRP channels / calcium ion transmembrane import into cytosol / cellular response to cold / intracellularly gated calcium channel activity / detection of mechanical stimulus involved in sensory perception of pain / positive regulation of insulin secretion involved in cellular response to glucose stimulus / voltage-gated calcium channel activity / monoatomic ion transport / sensory perception of pain / response to cold / calcium ion transmembrane transport / calcium channel activity / cellular response to hydrogen peroxide / intracellular calcium ion homeostasis / cellular response to heat / channel activity / protein homotetramerization / cell surface receptor signaling pathway / apical plasma membrane / response to xenobiotic stimulus / axon / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.05 Å | |||||||||
 Authors Authors | Rohou A / Rouge L / Chen H | |||||||||
 Citation Citation |  Journal: J Med Chem / Year: 2021 Journal: J Med Chem / Year: 2021Title: Tetrahydrofuran-Based Transient Receptor Potential Ankyrin 1 (TRPA1) Antagonists: Ligand-Based Discovery, Activity in a Rodent Asthma Model, and Mechanism-of-Action via Cryogenic Electron Microscopy. Authors: Jack A Terrett / Huifen Chen / Daniel G Shore / Elisia Villemure / Robin Larouche-Gauthier / Martin Déry / Francis Beaumier / Léa Constantineau-Forget / Chantal Grand-Maître / Luce ...Authors: Jack A Terrett / Huifen Chen / Daniel G Shore / Elisia Villemure / Robin Larouche-Gauthier / Martin Déry / Francis Beaumier / Léa Constantineau-Forget / Chantal Grand-Maître / Luce Lépissier / Stéphane Ciblat / Claudio Sturino / Yong Chen / Baihua Hu / Aijun Lu / Yunli Wang / Andrew P Cridland / Stuart I Ward / David H Hackos / Rebecca M Reese / Shannon D Shields / Jun Chen / Alessia Balestrini / Lorena Riol-Blanco / Wyne P Lee / John Liu / Eric Suto / Xiumin Wu / Juan Zhang / Justin Q Ly / Hank La / Kevin Johnson / Matt Baumgardner / Kang-Jye Chou / Alexis Rohou / Lionel Rougé / Brian S Safina / Steven Magnuson / Matthew Volgraf /     Abstract: Transient receptor potential ankyrin 1 (TRPA1) is a nonselective calcium-permeable ion channel highly expressed in the primary sensory neurons functioning as a polymodal sensor for exogenous and ...Transient receptor potential ankyrin 1 (TRPA1) is a nonselective calcium-permeable ion channel highly expressed in the primary sensory neurons functioning as a polymodal sensor for exogenous and endogenous stimuli and has generated widespread interest as a target for inhibition due to its implication in neuropathic pain and respiratory disease. Herein, we describe the optimization of a series of potent, selective, and orally bioavailable TRPA1 small molecule antagonists, leading to the discovery of a novel tetrahydrofuran-based linker. Given the balance of physicochemical properties and strong target engagement in a rat AITC-induced pain assay, compound was progressed into a guinea pig ovalbumin asthma model where it exhibited significant dose-dependent reduction of inflammatory response. Furthermore, the structure of the TRPA1 channel bound to compound was determined via cryogenic electron microscopy to a resolution of 3 Å, revealing the binding site and mechanism of action for this class of antagonists. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22490.map.gz emd_22490.map.gz | 4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22490-v30.xml emd-22490-v30.xml emd-22490.xml emd-22490.xml | 21.1 KB 21.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_22490.png emd_22490.png | 155.4 KB | ||
| Filedesc metadata |  emd-22490.cif.gz emd-22490.cif.gz | 7 KB | ||
| Others |  emd_22490_half_map_1.map.gz emd_22490_half_map_1.map.gz emd_22490_half_map_2.map.gz emd_22490_half_map_2.map.gz | 5.5 MB 5.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22490 http://ftp.pdbj.org/pub/emdb/structures/EMD-22490 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22490 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22490 | HTTPS FTP |
-Related structure data
| Related structure data |  7jupMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_22490.map.gz / Format: CCP4 / Size: 4.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22490.map.gz / Format: CCP4 / Size: 4.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Output from density modification in Phenix | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. generated in cubic-lattice coordinate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.262 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
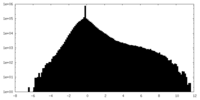
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: Half-map 1
| File | emd_22490_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half-map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
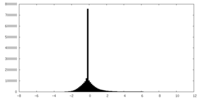
| Density Histograms |
-Half map: half map 2
| File | emd_22490_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
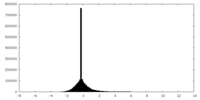
| Density Histograms |
- Sample components
Sample components
-Entire : TRPA1 bound by antagonist compound 21
| Entire | Name: TRPA1 bound by antagonist compound 21 |
|---|---|
| Components |
|
-Supramolecule #1: TRPA1 bound by antagonist compound 21
| Supramolecule | Name: TRPA1 bound by antagonist compound 21 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Transient receptor potential cation channel subfamily A member 1
| Macromolecule | Name: Transient receptor potential cation channel subfamily A member 1 type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 72.622125 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SPLHFAASYG RINTCQRLLQ DISDTRLLNE GDLHGMTPLH LAAKNGHDKV VQLLLKKGAL FLSDHNGWTA LHHASMGGYT QTMKVILDT NLKCTDRLDE DGNTALHFAA REGHAKAVAL LLSHNADIVL NKQQASFLHL ALHNKRKEVV LTIIRSKRWD E CLKIFSHN ...String: SPLHFAASYG RINTCQRLLQ DISDTRLLNE GDLHGMTPLH LAAKNGHDKV VQLLLKKGAL FLSDHNGWTA LHHASMGGYT QTMKVILDT NLKCTDRLDE DGNTALHFAA REGHAKAVAL LLSHNADIVL NKQQASFLHL ALHNKRKEVV LTIIRSKRWD E CLKIFSHN SPGNKCPITE MIEYLPECMK VLLDFCMLHS TEDKSCRDYY IEYNFKYLQC PLEFTKKTPT QDVIYEPLTA LN AMVQNNR IELLNHPVCK EYLLMKWLAY GFRAHMMNLG SYCLGLIPMT ILVVNIKPGM AFNSTGIINE TSDHSEILDT TNS YLIKTC MILVFLSSIF GYCKEAGQIF QQKRNYFMDI SNVLEWIIYT TGIIFVLPLF VEIPAHLQWQ CGAIAVYFYW MNFL LYLQR FENCGIFIVM LEVILKTLLR STVVFIFLLL AFGLSFYILL NLQDPFSSPL LSIIQTFSMM LGDINYRESF LEPYL RNEL AHPVLSFAQL VSFTIFVPIV LMNLLIGLAV GDIADVQKHA SLKRIAMQVE LHTSLEKKLP LWFLRKVDQK STIVYP NKP RSGGMLFHIF CFLFCTGEIR QEIPNADKSL EMEILKQKYR LKDLTFLLEK QHELIKLIIQ KMEIISET UniProtKB: Transient receptor potential cation channel subfamily A member 1 |
-Macromolecule #2: 1-({3-[(3R,5R)-5-(4-fluorophenyl)oxolan-3-yl]-1,2,4-oxadiazol-5-y...
| Macromolecule | Name: 1-({3-[(3R,5R)-5-(4-fluorophenyl)oxolan-3-yl]-1,2,4-oxadiazol-5-yl}methyl)-7-methyl-1,7-dihydro-6H-purin-6-one type: ligand / ID: 2 / Number of copies: 4 / Formula: VKM |
|---|---|
| Molecular weight | Theoretical: 396.375 Da |
| Chemical component information |  ChemComp-VKM: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.33 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8.2 Component:
| ||||||||||||
| Grid | Model: UltrAuFoil R2/2 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY / Support film - Film thickness: 25 / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: OTHER / Pretreatment - Pressure: 0.1 kPa / Details: Solarus plasma cleaner | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV Details: Triple blot. Put blot in vitroblot Apply 3.5ul to grid, wait 30sec, blot manually Apply 3.5ul to grid, wait 30sec, blot manually, apply final 3.5ul, final blot by vitrobot (3.5s). |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-40 / Number grids imaged: 1 / Number real images: 8915 / Average exposure time: 1.6 sec. / Average electron dose: 41.8 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal magnification: 165000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller













 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)