[English] 日本語
 Yorodumi
Yorodumi- EMDB-17580: Cryo-EM structure of a pre-dimerized murine IL-12 complete extrac... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of a pre-dimerized murine IL-12 complete extracellular signaling complex (Class 1), obtained after local refinement. | |||||||||
 Map data Map data | Main map: Sharpened map of the murine IL12:IL12Rbeta1-DAPK1:IL12Rbeta2-Calmodulin complex (Class 1, local refinement) used for model refinement in Phenix. Additional map: deepEMhancer sharpened map. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Complex / Cytokine / Receptor / SIGNALING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationinterleukin-12 beta subunit binding / Interleukin-12 signaling / Interleukin-23 signaling / interleukin-23 receptor binding / Interleukin-35 Signalling / interleukin-12 alpha subunit binding / interleukin-12 complex / interleukin-23 complex / T-helper 1 cell activation / natural killer cell activation involved in immune response ...interleukin-12 beta subunit binding / Interleukin-12 signaling / Interleukin-23 signaling / interleukin-23 receptor binding / Interleukin-35 Signalling / interleukin-12 alpha subunit binding / interleukin-12 complex / interleukin-23 complex / T-helper 1 cell activation / natural killer cell activation involved in immune response / positive regulation of natural killer cell mediated cytotoxicity directed against tumor cell target / negative regulation of vascular endothelial growth factor signaling pathway / negative regulation of blood vessel endothelial cell proliferation involved in sprouting angiogenesis / cellular response to hydroperoxide / positive regulation of T-helper 1 type immune response / regulation of response to tumor cell / positive regulation of autophagic cell death / DAPK1-calmodulin complex / positive regulation of smooth muscle cell apoptotic process / interleukin-12 receptor binding / T-helper cell differentiation / interleukin-23 receptor complex / defense response to tumor cell / natural killer cell activation / Caspase activation via Dependence Receptors in the absence of ligand / positive regulation of osteoclast differentiation / interleukin-12-mediated signaling pathway / negative regulation of interleukin-17 production / positive regulation of NK T cell proliferation / calcium/calmodulin-dependent protein kinase activity / regulation of NMDA receptor activity / response to UV-B / CaM pathway / positive regulation of natural killer cell proliferation / positive regulation of granulocyte macrophage colony-stimulating factor production / Cam-PDE 1 activation / cytokine receptor activity / Sodium/Calcium exchangers / syntaxin-1 binding / Calmodulin induced events / Reduction of cytosolic Ca++ levels / positive regulation of T cell differentiation / CREB1 phosphorylation through the activation of CaMKII/CaMKK/CaMKIV cascasde / Activation of Ca-permeable Kainate Receptor / Loss of phosphorylation of MECP2 at T308 / CREB1 phosphorylation through the activation of Adenylate Cyclase / PKA activation / negative regulation of high voltage-gated calcium channel activity / CaMK IV-mediated phosphorylation of CREB / Glycogen breakdown (glycogenolysis) / positive regulation of cyclic-nucleotide phosphodiesterase activity / organelle localization by membrane tethering / negative regulation of calcium ion export across plasma membrane / CLEC7A (Dectin-1) induces NFAT activation / autophagosome membrane docking / mitochondrion-endoplasmic reticulum membrane tethering / Activation of RAC1 downstream of NMDARs / regulation of cardiac muscle cell action potential / negative regulation of interleukin-10 production / : / positive regulation of ryanodine-sensitive calcium-release channel activity / regulation of cell communication by electrical coupling involved in cardiac conduction / Synthesis of IP3 and IP4 in the cytosol / defense response to protozoan / negative regulation of peptidyl-threonine phosphorylation / positive regulation of activated T cell proliferation / Negative regulation of NMDA receptor-mediated neuronal transmission / Phase 0 - rapid depolarisation / cytokine binding / Unblocking of NMDA receptors, glutamate binding and activation / negative regulation of ryanodine-sensitive calcium-release channel activity / protein phosphatase activator activity / positive regulation of interleukin-17 production / RHO GTPases activate PAKs / Ion transport by P-type ATPases / : / Uptake and function of anthrax toxins / Long-term potentiation / positive regulation of interleukin-10 production / Calcineurin activates NFAT / Regulation of MECP2 expression and activity / catalytic complex / DARPP-32 events / negative regulation of protein secretion / detection of calcium ion / regulation of cardiac muscle contraction / extrinsic apoptotic signaling pathway via death domain receptors / Smooth Muscle Contraction / regulation of ryanodine-sensitive calcium-release channel activity / RHO GTPases activate IQGAPs / immunoglobulin mediated immune response / regulation of cardiac muscle contraction by regulation of the release of sequestered calcium ion / calcium channel inhibitor activity / cellular response to interferon-beta / positive regulation of defense response to virus by host / eNOS activation / Protein methylation / coreceptor activity / voltage-gated potassium channel complex / positive regulation of autophagy Similarity search - Function | |||||||||
| Biological species |  | |||||||||
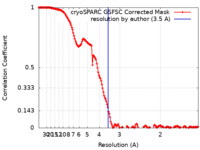
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Felix J / Bloch Y / Savvides SN | |||||||||
| Funding support |  Belgium, 2 items Belgium, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: Structures of complete extracellular receptor assemblies mediated by IL-12 and IL-23. Authors: Yehudi Bloch / Jan Felix / Romain Merceron / Mathias Provost / Royan Alipour Symakani / Robin De Backer / Elisabeth Lambert / Ahmad R Mehdipour / Savvas N Savvides /     Abstract: Cell-surface receptor complexes mediated by pro-inflammatory interleukin (IL)-12 and IL-23, both validated therapeutic targets, are incompletely understood due to the lack of structural insights into ...Cell-surface receptor complexes mediated by pro-inflammatory interleukin (IL)-12 and IL-23, both validated therapeutic targets, are incompletely understood due to the lack of structural insights into their complete extracellular assemblies. Furthermore, there is a paucity of structural details describing the IL-12-receptor interaction interfaces, in contrast to IL-23-receptor complexes. Here we report structures of fully assembled mouse IL-12/human IL-23-receptor complexes comprising the complete extracellular segments of the cognate receptors determined by electron cryo-microscopy. The structures reveal key commonalities but also surprisingly diverse features. Most notably, whereas IL-12 and IL-23 both utilize a conspicuously presented aromatic residue on their α-subunit as a hotspot to interact with the N-terminal Ig domain of their high-affinity receptors, only IL-12 juxtaposes receptor domains proximal to the cell membrane. Collectively, our findings will help to complete our understanding of cytokine-mediated assemblies of tall cytokine receptors and will enable a cytokine-specific interrogation of IL-12/IL-23 signaling in physiology and disease. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17580.map.gz emd_17580.map.gz | 166 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17580-v30.xml emd-17580-v30.xml emd-17580.xml emd-17580.xml | 26.1 KB 26.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17580_fsc.xml emd_17580_fsc.xml | 14.6 KB | Display |  FSC data file FSC data file |
| Images |  emd_17580.png emd_17580.png | 68.5 KB | ||
| Masks |  emd_17580_msk_1.map emd_17580_msk_1.map emd_17580_msk_2.map emd_17580_msk_2.map | 325 MB 325 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-17580.cif.gz emd-17580.cif.gz | 8 KB | ||
| Others |  emd_17580_additional_1.map.gz emd_17580_additional_1.map.gz emd_17580_half_map_1.map.gz emd_17580_half_map_1.map.gz emd_17580_half_map_2.map.gz emd_17580_half_map_2.map.gz | 268.8 MB 301.3 MB 301.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17580 http://ftp.pdbj.org/pub/emdb/structures/EMD-17580 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17580 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17580 | HTTPS FTP |
-Validation report
| Summary document |  emd_17580_validation.pdf.gz emd_17580_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_17580_full_validation.pdf.gz emd_17580_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_17580_validation.xml.gz emd_17580_validation.xml.gz | 23 KB | Display | |
| Data in CIF |  emd_17580_validation.cif.gz emd_17580_validation.cif.gz | 29.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17580 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17580 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17580 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-17580 | HTTPS FTP |
-Related structure data
| Related structure data |  8pb1MC  8c7mC  8cr5C  8cr6C  8cr8C  8odxC  8odzC  8oe0C  8oe4C  8ppmC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_17580.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17580.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Main map: Sharpened map of the murine IL12:IL12Rbeta1-DAPK1:IL12Rbeta2-Calmodulin complex (Class 1, local refinement) used for model refinement in Phenix. Additional map: deepEMhancer sharpened map. | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.755 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_17580_msk_1.map emd_17580_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
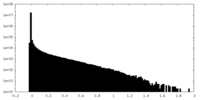
| Density Histograms |
-Mask #2
| File |  emd_17580_msk_2.map emd_17580_msk_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: DeepEMhancer sharpened map of the murine IL12:IL12Rbeta1-DAPK1:IL12Rbeta2-Calmodulin complex...
| File | emd_17580_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | DeepEMhancer sharpened map of the murine IL12:IL12Rbeta1-DAPK1:IL12Rbeta2-Calmodulin complex (Class 1, local refinement) used for model building in Coot and visualization. | ||||||||||||
| Projections & Slices |
| ||||||||||||
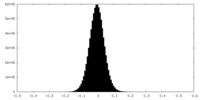
| Density Histograms |
-Half map: Half map 1 of the murine IL12:IL12Rbeta1-DAPK1:IL12Rbeta2-Calmodulin complex...
| File | emd_17580_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 of the murine IL12:IL12Rbeta1-DAPK1:IL12Rbeta2-Calmodulin complex (Class 1), obtained after local refinement. | ||||||||||||
| Projections & Slices |
| ||||||||||||
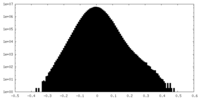
| Density Histograms |
-Half map: Half map 2 of the murine IL12:IL12Rbeta1-DAPK1:IL12Rbeta2-Calmodulin complex...
| File | emd_17580_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 of the murine IL12:IL12Rbeta1-DAPK1:IL12Rbeta2-Calmodulin complex (Class 1), obtained after local refinement. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Murine IL-12 in complex with mIL-12Rbeta1-DAPK1 and mIL-12Rbeta2-...
| Entire | Name: Murine IL-12 in complex with mIL-12Rbeta1-DAPK1 and mIL-12Rbeta2-Calmodulin. |
|---|---|
| Components |
|
-Supramolecule #1: Murine IL-12 in complex with mIL-12Rbeta1-DAPK1 and mIL-12Rbeta2-...
| Supramolecule | Name: Murine IL-12 in complex with mIL-12Rbeta1-DAPK1 and mIL-12Rbeta2-Calmodulin. type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 216 KDa |
-Macromolecule #1: Interleukin-12 subunit alpha
| Macromolecule | Name: Interleukin-12 subunit alpha / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 25.927496 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: RVIPVSGPAR CLSQSRNLLK TTDDMVKTAR EKLKHYSCTA EDIDHEDITR DQTSTLKTCL PLELHKNESC LATRETSSTT RGSCLPPQK TSLMMTLCLG SIYEDLKMYQ TEFQAINAAL QNHNHQQIIL DKGMLVAIDE LMQSLNHNGE TLRQKPPVGE A DPYRVKMK ...String: RVIPVSGPAR CLSQSRNLLK TTDDMVKTAR EKLKHYSCTA EDIDHEDITR DQTSTLKTCL PLELHKNESC LATRETSSTT RGSCLPPQK TSLMMTLCLG SIYEDLKMYQ TEFQAINAAL QNHNHQQIIL DKGMLVAIDE LMQSLNHNGE TLRQKPPVGE A DPYRVKMK LCILLHAFST RVVTINRVMG YLSSAGTSDE VDGGSGGSGL NDIFEAQKIE WHEGRTKHHH HHH UniProtKB: Interleukin-12 subunit alpha |
-Macromolecule #2: Interleukin-12 subunit beta
| Macromolecule | Name: Interleukin-12 subunit beta / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 35.83732 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MWELEKDVYV VEVDWTPDAP GETVNLTCDT PEEDDITWTS DQRHGVIGSG KTLTITVKEF LDAGQYTCHK GGETLSHSHL LLHKKENGI WSTEILKNFK NKTFLKCEAP NYSGRFTCSW LVQRNMDLKF NIKSSSSSPD SRAVTCGMAS LSAEKVTLDQ R DYEKYSVS ...String: MWELEKDVYV VEVDWTPDAP GETVNLTCDT PEEDDITWTS DQRHGVIGSG KTLTITVKEF LDAGQYTCHK GGETLSHSHL LLHKKENGI WSTEILKNFK NKTFLKCEAP NYSGRFTCSW LVQRNMDLKF NIKSSSSSPD SRAVTCGMAS LSAEKVTLDQ R DYEKYSVS CQEDVTCPTA EETLPIELAL EARQQNKYEN YSTSFFIRDI IKPDPPKNLQ MKPLKNSQVE VSWEYPDSWS TP HSYFSLK FFVRIQRKKE KMKETEEGCN QKGAFLVEKT STEVQCKGGN VCVQAQDRYY NSSCSKWACV PCRVRS UniProtKB: Interleukin-12 subunit beta |
-Macromolecule #3: Interleukin-12 receptor subunit beta-1,Death-associated protein k...
| Macromolecule | Name: Interleukin-12 receptor subunit beta-1,Death-associated protein kinase 1 type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO / EC number: non-specific serine/threonine protein kinase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 63.789156 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QLGASGPGDG CCVEKTSFPE GASGSPLGPR NLSCYRVSKT DYECSWQYDG PEDNVSHVLW CCFVPPNHTH TGQERCRYFS SGPDRTVQF WEQDGIPVLS KVNFWVESRL GNRTMKSQKI SQYLYNWTKT TPPLGHIKVS QSHRQLRMDW NVSEEAGAEV Q FRRRMPTT ...String: QLGASGPGDG CCVEKTSFPE GASGSPLGPR NLSCYRVSKT DYECSWQYDG PEDNVSHVLW CCFVPPNHTH TGQERCRYFS SGPDRTVQF WEQDGIPVLS KVNFWVESRL GNRTMKSQKI SQYLYNWTKT TPPLGHIKVS QSHRQLRMDW NVSEEAGAEV Q FRRRMPTT NWTLGDCGPQ VNSGSGVLGD IRGSMSESCL CPSENMAQEI QIRRRRRLSS GAPGGPWSDW SMPVCVPPEV LP QAKIKFL VEPLNQGGRR RLTMQGQSPQ LAVPEGCRGR PGAQVKKHLV LVRMLSCRCQ AQTSKTVPLG KKLNLSGATY DLN VLAKTR FGRSTIQKWH LPAQELTETR ALNVSVGGNM TSMQWAAQAP GTTYCLEWQP WFQHRNHTHC TLIVPEEEDP AKMV THSWS SKPTLEQEEC YRITVFASKN PKNPMLWATV LSSYYFGGNA SRAGTPRHVS VRNQTGDSVS VEWTASQLST CPGVL TQYV VRCEAEDGAW ESEWLVPPTK TQVTLDGLRS RVMYKVQVRA DTARLPGAWS HPQRFSFEGT GGSGGSGGAA RKKWKQ SVR LISLCQRLS UniProtKB: Interleukin-12 receptor subunit beta-1, Death-associated protein kinase 1 |
-Macromolecule #4: Interleukin-12 receptor subunit beta-2,Calmodulin-1
| Macromolecule | Name: Interleukin-12 receptor subunit beta-2,Calmodulin-1 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 85.723344 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: NIDVCKLGTV TVQPAPVIPL GSAANISCSL NPKQGCSHYP SSNELILLKF VNDVLVENLH GKKVHDHTGH SSTFQVTNLS LGMTLFVCK LNCSNSQKKP PVPVCGVEIS VGVAPEPPQN ISCVQEGENG TVACSWNSGK VTYLKTNYTL QLSGPNNLTC Q KQCFSDNR ...String: NIDVCKLGTV TVQPAPVIPL GSAANISCSL NPKQGCSHYP SSNELILLKF VNDVLVENLH GKKVHDHTGH SSTFQVTNLS LGMTLFVCK LNCSNSQKKP PVPVCGVEIS VGVAPEPPQN ISCVQEGENG TVACSWNSGK VTYLKTNYTL QLSGPNNLTC Q KQCFSDNR QNCNRLDLGI NLSPDLAESR FIVRVTAIND LGNSSSLPHT FTFLDIVIPL PPWDIRINFL NASGSRGTLQ WE DEGQVVL NQLRYQPLNS TSWNMVNATN AKGKYDLRDL RPFTEYEFQI SSKLHLSGGS WSNWSESLRT RTPEEEPVGI LDI WYMKQD IDYDRQQISL FWKSLNPSEA RGKILHYQVT LQEVTKKTTL QNTTRHTSWT RVIPRTGAWT ASVSAANSKG ASAP THINI VDLCGTGLLA PHQVSAKSEN MDNILVTWQP PKKADSAVRE YIVEWRALQP GSITKFPPHW LRIPPDNMSA LISEN IKPY ICYEIRVHAL SESQGGCSSI RGDSKHKAPV SGPHITAITE KKERLFISWT HIPFPEQRGC ILHYRIYWKE RDSTAQ PEL CEIQYRRSQN SHPISSLQPR VTYVLWMTAV TAAGESPQGN EREFCPQGKA NGTGGSGGSG GLTEEQIAEF KEAFSLF DK DGDGTITTKE LGTVMRSLGQ NPTEAELQDM INEVDADGNG TIDFPEFLTM MARKMKDTDS EEEIREAFRV FDKDGNGY I SAAELRHVMT NLGEKLTDEE VDEMIREADI DGDGQVNYEE FVQMMTAK UniProtKB: Interleukin-12 receptor subunit beta-2, Calmodulin-1 |
-Macromolecule #6: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 6 / Number of copies: 4 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #7: water
| Macromolecule | Name: water / type: ligand / ID: 7 / Number of copies: 1 / Formula: HOH |
|---|---|
| Molecular weight | Theoretical: 18.015 Da |
| Chemical component information |  ChemComp-HOH: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 Component:
Details: HEPES-buffered saline (HBS) with added calcium chloride: 25 mM HEPES, pH 7.4, 150 mM NaCl, 5 mM CaCl | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Instrument: LEICA PLUNGER / Details: Leica EM GP2, 5 s. blotting time.. |
- Electron microscopy
Electron microscopy
| Microscope | JEOL CRYO ARM 300 |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number real images: 8145 / Average exposure time: 3.37 sec. / Average electron dose: 61.8 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 60000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-8pb1: |
 Movie
Movie Controller
Controller









































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)