[English] 日本語
 Yorodumi
Yorodumi- EMDB-12037: Ubiquitin ligation to F-box protein substrates by SCF-RBR E3-E3 s... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12037 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Ubiquitin ligation to F-box protein substrates by SCF-RBR E3-E3 super-assembly: NEDD8-CUL1-RBX1-SKP1-SKP2-CKSHS1-Cyclin A-CDK2-p27-UBE2L3~Ub~ARIH1. Transition State 1 | |||||||||
 Map data Map data | TS1 SCF SKP2- Composite map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ubiquitin / ubiquitin ligase / E3 ligase / F-box protein / RBR ligase / Cullin-RING-Ligase / CRL / SCF / NEDD8 / Post-translational modification / ubiquitylation / LIGASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationPKR/eIFalpha signaling / cyclin-dependent protein kinase regulator activity / regulation of lens fiber cell differentiation / negative regulation of cyclin-dependent protein kinase activity / negative regulation of cardiac muscle tissue regeneration / negative regulation of kinase activity / positive regulation of protein polyubiquitination / ubiquitin-like protein transferase activity / autophagic cell death / FOXO-mediated transcription of cell cycle genes ...PKR/eIFalpha signaling / cyclin-dependent protein kinase regulator activity / regulation of lens fiber cell differentiation / negative regulation of cyclin-dependent protein kinase activity / negative regulation of cardiac muscle tissue regeneration / negative regulation of kinase activity / positive regulation of protein polyubiquitination / ubiquitin-like protein transferase activity / autophagic cell death / FOXO-mediated transcription of cell cycle genes / cell cycle phase transition / Parkin-FBXW7-Cul1 ubiquitin ligase complex / Lewy body / ubiquitin-protein transferase activator activity / RBR-type E3 ubiquitin transferase / F-box domain binding / negative regulation of epithelial cell proliferation involved in prostate gland development / cellular response to cell-matrix adhesion / negative regulation of cyclin-dependent protein serine/threonine kinase activity / : / cyclin A2-CDK1 complex / Aberrant regulation of mitotic exit in cancer due to RB1 defects / regulation of cell cycle G1/S phase transition / PcG protein complex / cell cycle G1/S phase transition / cellular response to luteinizing hormone stimulus / regulation of exit from mitosis / negative regulation of epithelial cell apoptotic process / cullin-RING-type E3 NEDD8 transferase / NEDD8 transferase activity / epithelial cell proliferation involved in prostate gland development / cullin-RING ubiquitin ligase complex / positive regulation of ubiquitin protein ligase activity / cyclin-dependent protein serine/threonine kinase inhibitor activity / Transcription of E2F targets under negative control by p107 (RBL1) and p130 (RBL2) in complex with HDAC1 / Cul7-RING ubiquitin ligase complex / cellular response to leptin stimulus / ubiquitin-dependent protein catabolic process via the C-end degron rule pathway / maintenance of protein location in nucleus / cellular response to chemical stress / Loss of Function of FBXW7 in Cancer and NOTCH1 Signaling / ubiquitin ligase activator activity / regulation of cyclin-dependent protein serine/threonine kinase activity / RHO GTPases activate CIT / male pronucleus / positive regulation of protein autoubiquitination / cyclin-dependent protein serine/threonine kinase activator activity / RNA polymerase II transcription initiation surveillance / nuclear export / female pronucleus / protein K11-linked ubiquitination / protein neddylation / negative regulation of mitotic cell cycle / ubiquitin conjugating enzyme binding / cellular response to cocaine / Modulation of host responses by IFN-stimulated genes / AKT phosphorylates targets in the cytosol / NEDD8 ligase activity / epithelial cell apoptotic process / response to glucagon / VCB complex / negative regulation of response to oxidative stress / cellular response to antibiotic / : / cyclin-dependent protein serine/threonine kinase regulator activity / Cul5-RING ubiquitin ligase complex / positive regulation of DNA biosynthetic process / cellular response to glucocorticoid stimulus / molecular function inhibitor activity / SCF ubiquitin ligase complex / ubiquitin-ubiquitin ligase activity / E2 ubiquitin-conjugating enzyme / negative regulation of type I interferon production / cellular response to lithium ion / SCF-dependent proteasomal ubiquitin-dependent protein catabolic process / Cul2-RING ubiquitin ligase complex / positive regulation of intracellular estrogen receptor signaling pathway / cellular response to steroid hormone stimulus / Cul3-RING ubiquitin ligase complex / cellular response to insulin-like growth factor stimulus / cyclin A1-CDK2 complex / cyclin E2-CDK2 complex / Cul4A-RING E3 ubiquitin ligase complex / cyclin E1-CDK2 complex / Cul4-RING E3 ubiquitin ligase complex / cyclin A2-CDK2 complex / positive regulation of DNA-templated DNA replication initiation / G2 Phase / Y chromosome / cyclin-dependent protein kinase activity / negative regulation of mitophagy / Phosphorylation of proteins involved in G1/S transition by active Cyclin E:Cdk2 complexes / positive regulation of heterochromatin formation / p53-Dependent G1 DNA Damage Response / Prolactin receptor signaling / Cul4B-RING E3 ubiquitin ligase complex / X chromosome / PTK6 Regulates Cell Cycle / ubiquitin ligase complex scaffold activity / RSV-host interactions Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Horn-Ghetko D / Prabu JR / Schulman BA | |||||||||
| Funding support |  Germany, 2 items Germany, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2021 Journal: Nature / Year: 2021Title: Ubiquitin ligation to F-box protein targets by SCF-RBR E3-E3 super-assembly. Authors: Daniel Horn-Ghetko / David T Krist / J Rajan Prabu / Kheewoong Baek / Monique P C Mulder / Maren Klügel / Daniel C Scott / Huib Ovaa / Gary Kleiger / Brenda A Schulman /    Abstract: E3 ligases are typically classified by hallmark domains such as RING and RBR, which are thought to specify unique catalytic mechanisms of ubiquitin transfer to recruited substrates. However, rather ...E3 ligases are typically classified by hallmark domains such as RING and RBR, which are thought to specify unique catalytic mechanisms of ubiquitin transfer to recruited substrates. However, rather than functioning individually, many neddylated cullin-RING E3 ligases (CRLs) and RBR-type E3 ligases in the ARIH family-which together account for nearly half of all ubiquitin ligases in humans-form E3-E3 super-assemblies. Here, by studying CRLs in the SKP1-CUL1-F-box (SCF) family, we show how neddylated SCF ligases and ARIH1 (an RBR-type E3 ligase) co-evolved to ubiquitylate diverse substrates presented on various F-box proteins. We developed activity-based chemical probes that enabled cryo-electron microscopy visualization of steps in E3-E3 ubiquitylation, initiating with ubiquitin linked to the E2 enzyme UBE2L3, then transferred to the catalytic cysteine of ARIH1, and culminating in ubiquitin linkage to a substrate bound to the SCF E3 ligase. The E3-E3 mechanism places the ubiquitin-linked active site of ARIH1 adjacent to substrates bound to F-box proteins (for example, substrates with folded structures or limited length) that are incompatible with previously described conventional RING E3-only mechanisms. The versatile E3-E3 super-assembly may therefore underlie widespread ubiquitylation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12037.map.gz emd_12037.map.gz | 75.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12037-v30.xml emd-12037-v30.xml emd-12037.xml emd-12037.xml | 32 KB 32 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_12037.png emd_12037.png | 39.7 KB | ||
| Filedesc metadata |  emd-12037.cif.gz emd-12037.cif.gz | 8.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12037 http://ftp.pdbj.org/pub/emdb/structures/EMD-12037 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12037 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12037 | HTTPS FTP |
-Validation report
| Summary document |  emd_12037_validation.pdf.gz emd_12037_validation.pdf.gz | 392.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_12037_full_validation.pdf.gz emd_12037_full_validation.pdf.gz | 392.2 KB | Display | |
| Data in XML |  emd_12037_validation.xml.gz emd_12037_validation.xml.gz | 6.8 KB | Display | |
| Data in CIF |  emd_12037_validation.cif.gz emd_12037_validation.cif.gz | 7.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12037 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12037 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12037 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12037 | HTTPS FTP |
-Related structure data
| Related structure data |  7b5lMC  7b5mC  7b5nC  7b5rC  7b5sC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12037.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12037.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | TS1 SCF SKP2- Composite map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.09 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : NEDD8-CUL1-RBX1-SKP1-SKP2-CKSHS1-Cyclin A-CDK2-p27-UBE2L3~Ub~ARIH...
+Supramolecule #1: NEDD8-CUL1-RBX1-SKP1-SKP2-CKSHS1-Cyclin A-CDK2-p27-UBE2L3~Ub~ARIH...
+Supramolecule #2: NEDD8-SKP1-SKP2-CKSHS1-Cyclin A-CDK2-p27-UBE2L3~Ub~ARIH1. Transit...
+Supramolecule #3: Cullin-1, E3 ubiquitin-protein ligase RBX1
+Macromolecule #1: Cullin-1
+Macromolecule #2: E3 ubiquitin-protein ligase ARIH1
+Macromolecule #3: S-phase kinase-associated protein 2
+Macromolecule #4: Cyclin-dependent kinases regulatory subunit 1
+Macromolecule #5: S-phase kinase-associated protein 1
+Macromolecule #6: Polyubiquitin-C
+Macromolecule #7: NEDD8
+Macromolecule #8: E3 ubiquitin-protein ligase RBX1
+Macromolecule #9: Ubiquitin-conjugating enzyme E2 L3
+Macromolecule #10: Cyclin-dependent kinase 2
+Macromolecule #11: Cyclin-A2
+Macromolecule #12: Cyclin-dependent kinase inhibitor 1B
+Macromolecule #13: ZINC ION
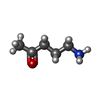
+Macromolecule #14: 5-azanylpentan-2-one
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 70.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


















































 X (Sec.)
X (Sec.) Y (Row.)
Y (Row.) Z (Col.)
Z (Col.)





















 Trichoplusia ni (cabbage looper)
Trichoplusia ni (cabbage looper)