[English] 日本語
 Yorodumi
Yorodumi- EMDB-11490: Human mTOR complex 2 with additional density close to the mLST8 -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-11490 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human mTOR complex 2 with additional density close to the mLST8 | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 5.2 Å | ||||||||||||
 Authors Authors | Scaiola A / Mangia F / Imseng S / Boehringer D / Ban N / Maier T | ||||||||||||
| Funding support |  Switzerland, 3 items Switzerland, 3 items
| ||||||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2020 Journal: Sci Adv / Year: 2020Title: The 3.2-Å resolution structure of human mTORC2. Authors: Alain Scaiola / Francesca Mangia / Stefan Imseng / Daniel Boehringer / Karolin Berneiser / Mitsugu Shimobayashi / Edward Stuttfeld / Michael N Hall / Nenad Ban / Timm Maier /  Abstract: The protein kinase mammalian target of rapamycin (mTOR) is the central regulator of cell growth. Aberrant mTOR signaling is linked to cancer, diabetes, and neurological disorders. mTOR exerts its ...The protein kinase mammalian target of rapamycin (mTOR) is the central regulator of cell growth. Aberrant mTOR signaling is linked to cancer, diabetes, and neurological disorders. mTOR exerts its functions in two distinct multiprotein complexes, mTORC1 and mTORC2. Here, we report a 3.2-Å resolution cryo-EM reconstruction of mTORC2. It reveals entangled folds of the defining Rictor and the substrate-binding SIN1 subunits, identifies the carboxyl-terminal domain of Rictor as the source of the rapamycin insensitivity of mTORC2, and resolves mechanisms for mTORC2 regulation by complex destabilization. Two previously uncharacterized small-molecule binding sites are visualized, an inositol hexakisphosphate (InsP6) pocket in mTOR and an mTORC2-specific nucleotide binding site in Rictor, which also forms a zinc finger. Structural and biochemical analyses suggest that InsP6 and nucleotide binding do not control mTORC2 activity directly but rather have roles in folding or ternary interactions. These insights provide a firm basis for studying mTORC2 signaling and for developing mTORC2-specific inhibitors. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_11490.map.gz emd_11490.map.gz | 117.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-11490-v30.xml emd-11490-v30.xml emd-11490.xml emd-11490.xml | 22.1 KB 22.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_11490.png emd_11490.png | 33.1 KB | ||
| Masks |  emd_11490_msk_1.map emd_11490_msk_1.map | 125 MB |  Mask map Mask map | |
| Others |  emd_11490_half_map_1.map.gz emd_11490_half_map_1.map.gz emd_11490_half_map_2.map.gz emd_11490_half_map_2.map.gz | 115.8 MB 115.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-11490 http://ftp.pdbj.org/pub/emdb/structures/EMD-11490 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11490 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-11490 | HTTPS FTP |
-Validation report
| Summary document |  emd_11490_validation.pdf.gz emd_11490_validation.pdf.gz | 662 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_11490_full_validation.pdf.gz emd_11490_full_validation.pdf.gz | 661.1 KB | Display | |
| Data in XML |  emd_11490_validation.xml.gz emd_11490_validation.xml.gz | 12.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11490 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11490 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11490 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-11490 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_11490.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_11490.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.344 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
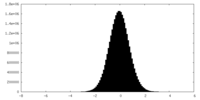
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_11490_msk_1.map emd_11490_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
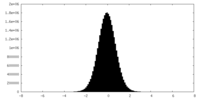
| Density Histograms |
-Half map: #1
| File | emd_11490_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_11490_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : human mTOR complex 2
| Entire | Name: human mTOR complex 2 |
|---|---|
| Components |
|
-Supramolecule #1: human mTOR complex 2
| Supramolecule | Name: human mTOR complex 2 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Molecular weight | Theoretical: 1.15 MDa |
-Macromolecule #1: Serine/threonine-protein kinase mTOR
| Macromolecule | Name: Serine/threonine-protein kinase mTOR / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO / EC number: non-specific serine/threonine protein kinase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Sequence | String: MLGTGPAAAT TAATTSSNVS VLQQFASGLK SRNEETRAKA AKELQHYVTM ELREMSQEES TRFYDQLNH HIFELVSSSD ANERKGGILA IASLIGVEGG NATRIGRFAN YLRNLLPSND P VVMEMASK AIGRLAMAGD TFTAEYVEFE VKRALEWLGA DRNEGRRHAA ...String: MLGTGPAAAT TAATTSSNVS VLQQFASGLK SRNEETRAKA AKELQHYVTM ELREMSQEES TRFYDQLNH HIFELVSSSD ANERKGGILA IASLIGVEGG NATRIGRFAN YLRNLLPSND P VVMEMASK AIGRLAMAGD TFTAEYVEFE VKRALEWLGA DRNEGRRHAA VLVLRELAIS VP TFFFQQV QPFFDNIFVA VWDPKQAIRE GAVAALRACL ILTTQREPKE MQKPQWYRHT FEE AEKGFD ETLAKEKGMN RDDRIHGALL ILNELVRISS MEGERLREEM EEITQQQLVH DKYC KDLMG FGTKPRHITP FTSFQAVQPQ QSNALVGLLG YSSHQGLMGF GTSPSPAKST LVESR CCRD LMEEKFDQVC QWVLKCRNSK NSLIQMTILN LLPRLAAFRP SAFTDTQYLQ DTMNHV LSC VKKEKERTAA FQALGLLSVA VRSEFKVYLP RVLDIIRAAL PPKDFAHKRQ KAMQVDA TV FTCISMLARA MGPGIQQDIK ELLEPMLAVG LSPALTAVLY DLSRQIPQLK KDIQDGLL K MLSLVLMHKP LRHPGMPKGL AHQLASPGLT TLPEASDVGS ITLALRTLGS FEFEGHSLT QFVRHCADHF LNSEHKEIRM EAARTCSRLL TPSIHLISGH AHVVSQTAVQ VVADVLSKLL VVGITDPDP DIRYCVLASL DERFDAHLAQ AENLQALFVA LNDQVFEIRE LAICTVGRLS S MNPAFVMP FLRKMLIQIL TELEHSGIGR IKEQSARMLG HLVSNAPRLI RPYMEPILKA LI LKLKDPD PDPNPGVINN VLATIGELAQ VSGLEMRKWV DELFIIIMDM LQDSSLLAKR QVA LWTLGQ LVASTGYVVE PYRKYPTLLE VLLNFLKTEQ NQGTRREAIR VLGLLGALDP YKHK VNIGM IDQSRDASAV SLSESKSSQD SSDYSTSEML VNMGNLPLDE FYPAVSMVAL MRIFR DQSL SHHHTMVVQA ITFIFKSLGL KCVQFLPQVM PTFLNVIRVC DGAIREFLFQ QLGMLV SFV KSHIRPYMDE IVTLMREFWV MNTSIQSTII LLIEQIVVAL GGEFKLYLPQ LIPHMLR VF MHDNSPGRIV SIKLLAAIQL FGANLDDYLH LLLPPIVKLF DAPEAPLPSR KAALETVD R LTESLDFTDY ASRIIHPIVR TLDQSPELRS TAMDTLSSLV FQLGKKYQIF IPMVNKVLV RHRINHQRYD VLICRIVKGY TLADEEEDPL IYQHRMLRSG QGDALASGPV ETGPMKKLHV STINLQKAW GAARRVSKDD WLEWLRRLSL ELLKDSSSPS LRSCWALAQA YNPMARDLFN A AFVSCWSE LNEDQQDELI RSIELALTSQ DIAEVTQTLL NLAEFMEHSD KGPLPLRDDN GI VLLGERA AKCRAYAKAL HYKELEFQKG PTPAILESLI SINNKLQQPE AAAGVLEYAM KHF GELEIQ ATWYEKLHEW EDALVAYDKK MDTNKDDPEL MLGRMRCLEA LGEWGQLHQQ CCEK WTLVN DETQAKMARM AAAAAWGLGQ WDSMEEYTCM IPRDTHDGAF YRAVLALHQD LFSLA QQCI DKARDLLDAE LTAMAGESYS RAYGAMVSCH MLSELEEVIQ YKLVPERREI IRQIWW ERL QGCQRIVEDW QKILMVRSLV VSPHEDMRTW LKYASLCGKS GRLALAHKTL VLLLGVD PS RQLDHPLPTV HPQVTYAYMK NMWKSARKID AFQHMQHFVQ TMQQQAQHAI ATEDQQHK Q ELHKLMARCF LKLGEWQLNL QGINESTIPK VLQYYSAATE HDRSWYKAWH AWAVMNFEA VLHYKHQNQA RDEKKKLRHA SGANITNATT AATTAATATT TASTEGSNSE SEAESTENSP TPSPLQKKV TEDLSKTLLM YTVPAVQGFF RSISLSRGNN LQDTLRVLTL WFDYGHWPDV N EALVEGVK AIQIDTWLQV IPQLIARIDT PRPLVGRLIH QLLTDIGRYH PQALIYPLTV AS KSTTTAR HNAANKILKN MCEHSNTLVQ QAMMVSEELI RVAILWHEMW HEGLEEASRL YFG ERNVKG MFEVLEPLHA MMERGPQTLK ETSFNQAYGR DLMEAQEWCR KYMKSGNVKD LTQA WDLYY HVFRRISKQL PQLTSLELQY VSPKLLMCRD LELAVPGTYD PNQPIIRIQS IAPSL QVIT SKQRPRKLTL MGSNGHEFVF LLKGHEDLRQ DERVMQLFGL VNTLLANDPT SLRKNL SIQ RYAVIPLSTN SGLIGWVPHC DTLHALIRDY REKKKILLNI EHRIMLRMAP DYDHLTL MQ KVEVFEHAVN NTAGDDLAKL LWLKSPSSEV WFDRRTNYTR SLAVMSMVGY ILGLGDRH P SNLMLDRLSG KILHIDFGDC FEVAMTREKF PEKIPFRLTR MLTNAMEVTG LDGNYRITC HTVMEVLREH KDSVMAVLEA FVYDPLLNWR LMDTNTKGNK RSRTRTDSYS AGQSVEILDG VELGEPAHK KTGTTVPESI HSFIGDGLVK PEALNKKAIQ IINRVRDKLT GRDFSHDDTL D VPTQVELL IKQATSHENL CQCYIGWCPF W |
-Macromolecule #2: Target of rapamycin complex subunit LST8
| Macromolecule | Name: Target of rapamycin complex subunit LST8 / type: protein_or_peptide / ID: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Sequence | String: MNTSPGTVGS DPVILATAGY DHTVRFWQAH SGICTRTVQH QDSQVNALEV TPDRSMIAAA GYQHIRMYD LNSNNPNPII SYDGVNKNIA SVGFHEDGRW MYTGGEDCTA RIWDLRSRNL Q CQRIFQVN APINCVCLHP NQAELIVGDQ SGAIHIWDLK TDHNEQLIPE ...String: MNTSPGTVGS DPVILATAGY DHTVRFWQAH SGICTRTVQH QDSQVNALEV TPDRSMIAAA GYQHIRMYD LNSNNPNPII SYDGVNKNIA SVGFHEDGRW MYTGGEDCTA RIWDLRSRNL Q CQRIFQVN APINCVCLHP NQAELIVGDQ SGAIHIWDLK TDHNEQLIPE PEVSITSAHI DP DASYMAA VNSTGNCYVW NLTGGIGDEV TQLIPKTKIP AHTRYALQCR FSPDSTLLAT CSA DQTCKI WRTSNFSLMT ELSIKSGNPG ESSRGWMWGC AFSGDSQYIV TASSDNLARL WCVE TGEIK REYGGHQKAV VCLAFNDSVL G |
-Macromolecule #3: Rapamycin-insensitive companion of mTOR
| Macromolecule | Name: Rapamycin-insensitive companion of mTOR / type: protein_or_peptide / ID: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Sequence | String: MAAIGRGRSL KNLRVRGRND SGEENVPLDL TREPSDNLRE ILQNVARLQG VSNMRKLGHL NNFTKLLCD IGHSEEKLGF HYEDIIICLR LALLNEAKEV RAAGLRALRY LIQDSSILQK V LKLKVDYL IARCIDIQQS NEVERTQALR LVRKMITVNA SLFPSSVTNS ...String: MAAIGRGRSL KNLRVRGRND SGEENVPLDL TREPSDNLRE ILQNVARLQG VSNMRKLGHL NNFTKLLCD IGHSEEKLGF HYEDIIICLR LALLNEAKEV RAAGLRALRY LIQDSSILQK V LKLKVDYL IARCIDIQQS NEVERTQALR LVRKMITVNA SLFPSSVTNS LIAVGNDGLQ ER DRMVRAC IAIICELALQ NPEVVALRGG LNTILKNVID CQLSRINEAL ITTILHLLNH PKT RQYVRA DVELERILAP YTDFHYRHSP DTAEGQLKED REARFLASKM GIIATFRSWA GIIN LCKPG NSGIQSLIGV LCIPNMEIRR GLLEVLYDIF RLPLPVVTEE FIEALLSVDP GRFQD SWRL SDGFVAAEAK TILPHRARSR PDLMDNYLAL ILSAFIRNGL LEGLVEVITN SDDHIS VRA TILLGELLHM ANTILPHSHS HHLHCLPTLM NMAASFDIPK EKRLRASAAL NCLKRFH EM KKRGPKPYSL HLDHIIQKAI ATHQKRDQYL RVQKDIFILK DTEEALLINL RDSQVLQH K ENLEWNWNLI GTILKWPNVN LRNYKDEQLH RFVRRLLYFY KPSSKLYANL DLDFAKAKQ LTVVGCQFTE FLLESEEDGQ GYLEDLVKDI VQWLNASSGM KPERSLQNNG LLTTLSQHYF LFIGTLSCH PHGVKMLEKC SVFQCLLNLC SLKNQDHLLK LTVSSLDYSR DGLARVILSK I LTAATDAC RLYATKHLRV LLRANVEFFN NWGIELLVTQ LHDKNKTISS EALDILDEAC ED KANLHAL IQMKPALSHL GDKGLLLLLR FLSIPKGFSY LNERGYVAKQ LEKWHREYNS KYV DLIEEQ LNEALTTYRK PVDGDNYVRR SNQRLQRPHV YLPIHLYGQL VHHKTGCHLL EVQN IITEL CRNVRTPDLD KWEEIKKLKA SLWALGNIGS SNWGLNLLQE ENVIPDILKL AKQCE VLSI RGTCVYVLGL IAKTKQGCDI LKCHNWDAVR HSRKHLWPVV PDDVEQLCNE LSSIPS TLS LNSESTSSRH NSESESVPSS MFILEDDRFG SSSTSTFFLD INEDTEPTFY DRSGPIK DK NSFPFFASSK LVKNRILNSL TLPNKKHRSS SDPKGGKLSS ESKTSNRRIR TLTEPSVD F NHSDDFTPIS TVQKTLQLET SFMGNKHIED TGSTPSIGEN DLKFTKNFGT ENHRENTSR ERLVVESSTS SHMKIRSQSF NTDTTTSGIS SMSSSPSRET VGVDATTMDT DCGSMSTVVS TKTIKTSHY LTPQSNHLSL SKSNSVSLVP PGSSHTLPRR AQSLKAPSIA TIKSLADCNF S YTSSRDAF GYATLKRLQQ QRMHPSLSHS EALASPAKDV LFTDTITMKA NSFESRLTPS RF MKALSYA SLDKEDLLSP INQNTLQRSS SVRSMVSSAT YGGSDDYIGL ALPVDINDIF QVK DIPYFQ TKNIPPHDDR GARAFAHDAG GLPSGTGGLV KNSFHLLRQQ MSLTEIMNSI HSDA SLFLE STEDTGLQEH TDDNCLYCVC IEILGFQPSN QLSAICSHSD FQDIPYSDWC EQTIH NPLE VVPSKFSGIS GCSDGVSQEG SASSTKSTEL LLGVKTIPDD TPMCRILLRK EVLRLV INL SSSVSTKCHE TGLLTIKEKY PQTFDDICLY SEVSHLLSHC TFRLPCRRFI QELFQDV QF LQMHEEAEAV LATPPKQPIV DTSAES |
-Macromolecule #4: Target of rapamycin complex 2 subunit MAPKAP1
| Macromolecule | Name: Target of rapamycin complex 2 subunit MAPKAP1 / type: protein_or_peptide / ID: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Sequence | String: (ACE)AFLDNPTII LAHIRQSHVT SDDTGMCEMV LIDHDVDLEK IHPPSMPGDS GSEIQGSNGE TQGYVYAQS VDITSSWDFG IRRRSNTAQR LERLRKERQN QIKCKNIQWK ERNSKQSAQE L KSLFEKKS LKEKPPISGK QSILSVRLEQ CPLQLNNPFN EYSKFDGKGH ...String: (ACE)AFLDNPTII LAHIRQSHVT SDDTGMCEMV LIDHDVDLEK IHPPSMPGDS GSEIQGSNGE TQGYVYAQS VDITSSWDFG IRRRSNTAQR LERLRKERQN QIKCKNIQWK ERNSKQSAQE L KSLFEKKS LKEKPPISGK QSILSVRLEQ CPLQLNNPFN EYSKFDGKGH VGTTATKKID VY LPLHSSQ DRLLPMTVVT MASARVQDLI GLICWQYTSE GREPKLNDNV SAYCLHIAED DGE VDTDFP PLDSNEPIHK FGFSTLALVE KYSSPGLTSK ESLFVRINAA HGFSLIQVDN TKVT MKEIL LKAVKRRKGS QKVSGPQYRL EKQSEPNVAV DLDSTLESQS AWEFCLVREN SSRAD GVFE EDSQIDIATV QDMLSSHHYK SFKVSMIHRL RFTTDVQLGI SGDKVEIDPV TNQKAS TKF WIKQKPISID SDLLCACDLA EEKSPSHAIF KLTYLSNHDY KHLYFESDAA TVNEIVL KV NYILESRAST ARADYFAQKQ RKLNRRTSFS FQKEKKSGQQ |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.37 mg/mL |
|---|---|
| Buffer | pH: 8.5 |
| Grid | Model: Quantifoil / Material: COPPER / Mesh: 400 / Support film - #0 - Film type ID: 1 / Support film - #0 - Material: CARBON / Support film - #0 - topology: HOLEY / Support film - #1 - Film type ID: 2 / Support film - #1 - Material: CARBON / Support film - #1 - topology: CONTINUOUS |
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 70.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 5.2 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 19920 |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)