+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-1044 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | Structure and gating mechanism of the acetylcholine receptor pore. | |||||||||
 マップデータ マップデータ | The map is of the membrane-spanning domain of the nicotinic acetylcholine receptor in the closed state, viewed from the synaptic cleft. The arrangement of subunits around the central axis, clockwise beginning from the bottom (closest to 0 on the y-axis) ia alpha, gamma, beta, delta. The Fourier terms were derived from tubular crystals having helical symmetry. They are of higher quality along the meridional (y-axis) direction than the equatorial direction (where the diffraction is weaker and there is additional noise associated with layer-line overlap. This has resulted in some asymmetry in the map, with the best direction being along the axis of the tube (y-axis). The map was obtained by averaging data from four helical families in real space, weighting each family approximately according to the number of receptors analysed. The actual weights were: 0.70 (-16,6); 0.30 (-15,7); 0.30 (-17,5); 0.25 (-18,6). As explained in the Reference, the dominating low resolution terms were weakened by subtracting a map of the structure with terms extending to only 15 Angstroms. THe weight used for the subtraction map was -0.88. The terms along the equator have also been included with a weight of 0.04, so that the densities corresponding to the alpha-helical segments are represented at about the same level throughout the thickness of the bilayer. | |||||||||
 試料 試料 |
| |||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報acetylcholine-gated channel complex / acetylcholine receptor signaling pathway / acetylcholine-gated monoatomic cation-selective channel activity / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / transmembrane signaling receptor activity / postsynaptic membrane / neuron projection 類似検索 - 分子機能 | |||||||||
| 生物種 |  | |||||||||
| 手法 | らせん対称体再構成法 / クライオ電子顕微鏡法 / ネガティブ染色法 / 解像度: 4.0 Å | |||||||||
 データ登録者 データ登録者 | Miyazawa A / Fujiyoshi Y / Unwin N | |||||||||
 引用 引用 |  ジャーナル: Nature / 年: 2003 ジャーナル: Nature / 年: 2003タイトル: Structure and gating mechanism of the acetylcholine receptor pore. 著者: Atsuo Miyazawa / Yoshinori Fujiyoshi / Nigel Unwin /  要旨: The nicotinic acetylcholine receptor controls electrical signalling between nerve and muscle cells by opening and closing a gated, membrane-spanning pore. Here we present an atomic model of the ...The nicotinic acetylcholine receptor controls electrical signalling between nerve and muscle cells by opening and closing a gated, membrane-spanning pore. Here we present an atomic model of the closed pore, obtained by electron microscopy of crystalline postsynaptic membranes. The pore is shaped by an inner ring of 5 alpha-helices, which curve radially to create a tapering path for the ions, and an outer ring of 15 alpha-helices, which coil around each other and shield the inner ring from the lipids. The gate is a constricting hydrophobic girdle at the middle of the lipid bilayer, formed by weak interactions between neighbouring inner helices. When acetylcholine enters the ligand-binding domain, it triggers rotations of the protein chains on opposite sides of the entrance to the pore. These rotations are communicated through the inner helices, and open the pore by breaking the girdle apart. | |||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_1044.map.gz emd_1044.map.gz | 472.7 KB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-1044-v30.xml emd-1044-v30.xml emd-1044.xml emd-1044.xml | 14.7 KB 14.7 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
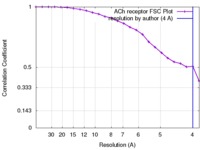
| FSC (解像度算出) |  emd_1044_fsc.xml emd_1044_fsc.xml | 1.9 KB | 表示 |  FSCデータファイル FSCデータファイル |
| 画像 |  1044.gif 1044.gif | 35.9 KB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-1044 http://ftp.pdbj.org/pub/emdb/structures/EMD-1044 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1044 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-1044 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_1044_validation.pdf.gz emd_1044_validation.pdf.gz | 229.8 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_1044_full_validation.pdf.gz emd_1044_full_validation.pdf.gz | 228.9 KB | 表示 | |
| XML形式データ |  emd_1044_validation.xml.gz emd_1044_validation.xml.gz | 5.3 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1044 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1044 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1044 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-1044 | HTTPS FTP |
-関連構造データ
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_1044.map.gz / 形式: CCP4 / 大きさ: 3.4 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_1044.map.gz / 形式: CCP4 / 大きさ: 3.4 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | The map is of the membrane-spanning domain of the nicotinic acetylcholine receptor in the closed state, viewed from the synaptic cleft. The arrangement of subunits around the central axis, clockwise beginning from the bottom (closest to 0 on the y-axis) ia alpha, gamma, beta, delta. The Fourier terms were derived from tubular crystals having helical symmetry. They are of higher quality along the meridional (y-axis) direction than the equatorial direction (where the diffraction is weaker and there is additional noise associated with layer-line overlap. This has resulted in some asymmetry in the map, with the best direction being along the axis of the tube (y-axis). The map was obtained by averaging data from four helical families in real space, weighting each family approximately according to the number of receptors analysed. The actual weights were: 0.70 (-16,6); 0.30 (-15,7); 0.30 (-17,5); 0.25 (-18,6). As explained in the Reference, the dominating low resolution terms were weakened by subtracting a map of the structure with terms extending to only 15 Angstroms. THe weight used for the subtraction map was -0.88. The terms along the equator have also been included with a weight of 0.04, so that the densities corresponding to the alpha-helical segments are represented at about the same level throughout the thickness of the bilayer. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
- 試料の構成要素
試料の構成要素
-全体 : Crystalline postsynaptic membrane from Torpedo marmorata electric...
| 全体 | 名称: Crystalline postsynaptic membrane from Torpedo marmorata electric organ |
|---|---|
| 要素 |
|
-超分子 #1000: Crystalline postsynaptic membrane from Torpedo marmorata electric...
| 超分子 | 名称: Crystalline postsynaptic membrane from Torpedo marmorata electric organ タイプ: sample / ID: 1000 集合状態: The acetylcholine receptors are hetero-pentamers composed of 2 alpha 1 beta 1 gamma and 1 delta subunit Number unique components: 2 |
|---|
-超分子 #1: postsynaptic membrane lipids
| 超分子 | 名称: postsynaptic membrane lipids / タイプ: organelle_or_cellular_component / ID: 1 / 組換発現: No / データベース: NCBI |
|---|---|
| 由来(天然) | 生物種:  |
-分子 #1: acetylcholine receptor
| 分子 | 名称: acetylcholine receptor / タイプ: protein_or_peptide / ID: 1 詳細: This is the MW of the glycosylated protein. The protein itself accounts for 258kD 集合状態: pentamer / 組換発現: No / データベース: NCBI |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 実験値: 290 KDa |
-実験情報
-構造解析
| 手法 | ネガティブ染色法, クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | らせん対称体再構成法 |
| 試料の集合状態 | filament |
- 試料調製
試料調製
| 緩衝液 | pH: 6.8 / 詳細: 100mM sodium cacodylate, 1mM CaCl2 |
|---|---|
| 染色 | タイプ: NEGATIVE / 詳細: no stains or fixatives used |
| グリッド | 詳細: holey carbon film made over 300 mesh copper grids. To minimise beam movement at the 4K imaging temperature, it was essential that the carbon films had a high electrical conductivity - ...詳細: holey carbon film made over 300 mesh copper grids. To minimise beam movement at the 4K imaging temperature, it was essential that the carbon films had a high electrical conductivity - achieved by evaporation of carbon in a high vacuum and pre-irradiation of the grids. |
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 90 % / チャンバー内温度: 100 K / 装置: HOMEMADE PLUNGER 詳細: Vitrification instrument: Home-built model. The plunging apparatus was contained in a bench-top fridge having a window made in the door. Wet air was continually bubbled into the fridge, which ...詳細: Vitrification instrument: Home-built model. The plunging apparatus was contained in a bench-top fridge having a window made in the door. Wet air was continually bubbled into the fridge, which was maintained at 4-8 deg. centigrade. 手法: The grid was first glow-discharged in the presence of amyl amine. The specimen was applied to the carbon-film side in 4.2ul droplets. Blotting was done from the other side, removing the ...手法: The grid was first glow-discharged in the presence of amyl amine. The specimen was applied to the carbon-film side in 4.2ul droplets. Blotting was done from the other side, removing the filter paper and plunging as soon as the paper and grid were observed to lose water-contact with each other - typically after 6 seconds. |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | JEOL KYOTO-3000SFF |
|---|---|
| 温度 | 最低: 4.2 K / 最高: 4.2 K / 平均: 4.2 K |
| アライメント法 | Legacy - 非点収差: correction on carbon film at 250,000 |
| 撮影 | カテゴリ: FILM / フィルム・検出器のモデル: KODAK SO-163 FILM / デジタル化 - スキャナー: OTHER / デジタル化 - サンプリング間隔: 5 µm / 実像数: 359 / 平均電子線量: 20 e/Å2 詳細: Scanning done with a point-source, flat-bed Joyce-Loebl microdensitometer, modified in-house Od range: 1 / ビット/ピクセル: 10 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 倍率(補正後): 36800 / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 1.3 mm / 最大 デフォーカス(公称値): 1.8 µm / 最小 デフォーカス(公称値): 0.8 µm / 倍率(公称値): 40000 |
| 試料ステージ | 試料ホルダー: top-entry / 試料ホルダーモデル: OTHER |
- 画像解析
画像解析
-原子モデル構築 1
| 詳細 | Interpretation of the experimental density map and model building into the densities were performed using O. The helical segments were fitted individually, using the protruding regions along the helical densities to identify the largest side chains. This allowed tentative assignments to be made of each amino acid according to the sequence, both along the helices and along the short connecting loops. These assignments were then validated for each subunit by checking their consistency with residues in equivalent positions around the pentamer. |
|---|---|
| 得られたモデル |  PDB-1oed: |
 ムービー
ムービー コントローラー
コントローラー