+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-0968 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of human BAF Base module | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationPositive Regulation of CDH1 Gene Transcription / Formation of the canonical BAF (cBAF) complex / Formation of the embryonic stem cell BAF (esBAF) complex / Formation of neuronal progenitor and neuronal BAF (npBAF and nBAF) / negative regulation of myeloid progenitor cell differentiation / single stranded viral RNA replication via double stranded DNA intermediate / positive regulation of glucose mediated signaling pathway / positive regulation of transcription of nucleolar large rRNA by RNA polymerase I / bBAF complex / neural retina development ...Positive Regulation of CDH1 Gene Transcription / Formation of the canonical BAF (cBAF) complex / Formation of the embryonic stem cell BAF (esBAF) complex / Formation of neuronal progenitor and neuronal BAF (npBAF and nBAF) / negative regulation of myeloid progenitor cell differentiation / single stranded viral RNA replication via double stranded DNA intermediate / positive regulation of glucose mediated signaling pathway / positive regulation of transcription of nucleolar large rRNA by RNA polymerase I / bBAF complex / neural retina development / npBAF complex / negative regulation of androgen receptor signaling pathway / nBAF complex / brahma complex / histone H3K14ac reader activity / EGR2 and SOX10-mediated initiation of Schwann cell myelination / blastocyst hatching / N-acetyltransferase activity / nucleosome array spacer activity / hepatocyte differentiation / GBAF complex / regulation of G0 to G1 transition / Tat protein binding / RNA polymerase I preinitiation complex assembly / XY body / RSC-type complex / ATP-dependent chromatin remodeler activity / host-mediated activation of viral transcription / cellular response to fatty acid / regulation of nucleotide-excision repair / nucleosome disassembly / germ cell nucleus / SWI/SNF complex / regulation of mitotic metaphase/anaphase transition / positive regulation of T cell differentiation / nuclear androgen receptor binding / nuclear chromosome / positive regulation of double-strand break repair / positive regulation of stem cell population maintenance / RUNX1 interacts with co-factors whose precise effect on RUNX1 targets is not known / Regulation of MITF-M-dependent genes involved in pigmentation / regulation of G1/S transition of mitotic cell cycle / negative regulation of cell differentiation / histone H4K16ac reader activity / positive regulation of myoblast differentiation / positive regulation of signal transduction by p53 class mediator / ATP-dependent activity, acting on DNA / positive regulation of Wnt signaling pathway / Chromatin modifying enzymes / DNA polymerase binding / neurogenesis / transcription initiation-coupled chromatin remodeling / Interleukin-7 signaling / nuclear receptor binding / transcription coregulator binding / transcription coregulator activity / positive regulation of cell differentiation / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / apoptotic signaling pathway / Formation of the beta-catenin:TCF transactivating complex / helicase activity / negative regulation of cell growth / DNA integration / kinetochore / positive regulation of miRNA transcription / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / nuclear matrix / fibrillar center / RMTs methylate histone arginines / p53 binding / transcription corepressor activity / nervous system development / positive regulation of cold-induced thermogenesis / histone binding / molecular adaptor activity / transcription coactivator activity / chromatin remodeling / hydrolase activity / signaling receptor binding / negative regulation of cell population proliferation / intracellular membrane-bounded organelle / negative regulation of DNA-templated transcription / positive regulation of cell population proliferation / apoptotic process / chromatin binding / centrosome / regulation of transcription by RNA polymerase II / chromatin / positive regulation of DNA-templated transcription / nucleolus / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / protein-containing complex / extracellular space / DNA binding / RNA binding / zinc ion binding / nucleoplasm / ATP binding / identical protein binding Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Shuang H / Zihan W / Yuan T / Zishuo Y / Jiali Y / Xinxin W / Jie L / Bijun L / Yanhui X | |||||||||
 Citation Citation |  Journal: Science / Year: 2020 Journal: Science / Year: 2020Title: Structure of nucleosome-bound human BAF complex. Authors: Shuang He / Zihan Wu / Yuan Tian / Zishuo Yu / Jiali Yu / Xinxin Wang / Jie Li / Bijun Liu / Yanhui Xu /  Abstract: Mammalian SWI/SNF family chromatin remodelers, BRG1/BRM-associated factor (BAF) and polybromo-associated BAF (PBAF), regulate chromatin structure and transcription, and their mutations are linked to ...Mammalian SWI/SNF family chromatin remodelers, BRG1/BRM-associated factor (BAF) and polybromo-associated BAF (PBAF), regulate chromatin structure and transcription, and their mutations are linked to cancers. The 3.7-angstrom-resolution cryo-electron microscopy structure of human BAF bound to the nucleosome reveals that the nucleosome is sandwiched by the base and the adenosine triphosphatase (ATPase) modules, which are bridged by the actin-related protein (ARP) module. The ATPase motor is positioned proximal to nucleosomal DNA and, upon ATP hydrolysis, engages with and pumps DNA along the nucleosome. The C-terminal α helix of SMARCB1, enriched in positively charged residues frequently mutated in cancers, mediates interactions with an acidic patch of the nucleosome. AT-rich interactive domain-containing protein 1A (ARID1A) and the SWI/SNF complex subunit SMARCC serve as a structural core and scaffold in the base module organization, respectively. Our study provides structural insights into subunit organization and nucleosome recognition of human BAF complex. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_0968.map.gz emd_0968.map.gz | 25.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-0968-v30.xml emd-0968-v30.xml emd-0968.xml emd-0968.xml | 14.4 KB 14.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_0968.png emd_0968.png | 5.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0968 http://ftp.pdbj.org/pub/emdb/structures/EMD-0968 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0968 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0968 | HTTPS FTP |
-Related structure data
| Related structure data |  6lthMC  0969C  0970C  0971C  0972C  0973C  0974C  6ltjC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_0968.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_0968.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
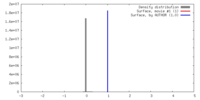
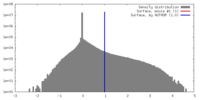
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Structure of nucleosome-bound human BAF Base module
| Entire | Name: Structure of nucleosome-bound human BAF Base module |
|---|---|
| Components |
|
-Supramolecule #1: Structure of nucleosome-bound human BAF Base module
| Supramolecule | Name: Structure of nucleosome-bound human BAF Base module / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#20 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Homo sapiens (human) / Recombinant cell: HEK293T Homo sapiens (human) / Recombinant cell: HEK293T |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Frames/image: 1-32 / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: OTHER |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| CTF correction | Software - Name: Gctf (ver. 1.06) |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 3.0 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC (ver. 2.12.4) / Number images used: 197606 |
| Initial angle assignment | Type: OTHER / Software - Name: cryoSPARC (ver. 2.12.4) |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD / Software - Name: RELION (ver. 3.0.8) |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-6lth: |
 Movie
Movie Controller
Controller

























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)