+Search query
-Structure paper
| Title | Noncanonical agonist-dependent and -independent arrestin recruitment of GPR1. |
|---|---|
| Journal, issue, pages | Science, Vol. 390, Issue 6775, Page eadt8794, Year 2025 |
| Publish date | Nov 20, 2025 |
 Authors Authors | Heng Cai / Xiaowen Lin / Lechen Zhao / Maozhou He / Jie Yu / Bingjie Zhang / Yuandi Ma / Xiaohua Chang / Yuxuan Tang / Tianyu Luo / Jie Jiang / Mengna Ma / Wenqi Song / Limin Ma / Xiaojing Chu / Cuiying Yi / Kun Chen / Shuo Han / Cen Xie / Wenqing Shui / Qiang Zhao / Ya Zhu / Beili Wu /  |
| PubMed Abstract | G protein (heterotrimeric guanine nucleotide-binding protein)-coupled receptors have diverse signaling properties with differential preferences for downstream pathways. Certain receptors, such as the ...G protein (heterotrimeric guanine nucleotide-binding protein)-coupled receptors have diverse signaling properties with differential preferences for downstream pathways. Certain receptors, such as the chemerin receptor GPR1, undergo arrestin-mediated internalization but weak G protein signaling. However, the mechanisms of this unusual signaling pattern and its physiological relevance are unclear. We report the structures of GPR1 bound to chemerin and β-arrestin 1 or β-arrestin 2 and an agonist-free GPR1-β-arrestin 1 complex. Upon agonist stimulation, the receptor binds the two arrestins in distinct interaction patterns, which may account for their differential cellular responses. Agonist-independent internalization was mediated by an inactive, constitutively phosphorylated GPR1 that accommodates β-arrestin 1 in an unconventional pocket together with a fatty acid, which potentially provides a basis for GPR1 modulating lipid accumulation in lipid-overloaded adipocytes. |
 External links External links |  Science / Science /  PubMed:41264711 PubMed:41264711 |
| Methods | EM (single particle) |
| Resolution | 2.9 - 3.5 Å |
| Structure data | EMDB-64610, PDB-9uyh: EMDB-64611, PDB-9uyi: EMDB-64612, PDB-9uyj: EMDB-64614, PDB-9uyl:  EMDB-64615: Cryo-EM structure of the G protein-coupled receptor 1 (GPR1) bound to chemerin and beta-arrestin 2 (focused refinement in chemerin and GPR1)  EMDB-64616: Cryo-EM structure of the G protein-coupled receptor 1 (GPR1) bound to chemerin and beta-arrestin 2 (focused refinement in beta-arrestin 2)  EMDB-64617: Cryo-EM structure of the G protein-coupled receptor 1 (GPR1) bound to chemerin and beta-arrestin 2 (consensus refinement) EMDB-64618, PDB-9uym: EMDB-64619, PDB-9uyn: |
| Chemicals |  ChemComp-Y01: 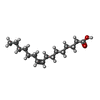 ChemComp-PAM: |
| Source |
|
 Keywords Keywords | MEMBRANE PROTEIN/IMMUNE SYSTEM / G protein-coupled receptor 1 / chemerin / beta-arrestin1 / signaling protein / MEMBRANE PROTEIN-IMMUNE SYSTEM complex / beta-arrestin 2 |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers















 homo sapiens (human)
homo sapiens (human) escherichia phage ecszw-2 (virus)
escherichia phage ecszw-2 (virus)