[English] 日本語
 Yorodumi
Yorodumi- EMDB-64619: Cryo-EM structure of the G protein-coupled receptor 1 (GPR1) boun... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the G protein-coupled receptor 1 (GPR1) bound to beta-arrestin 1 in ligand-free state | |||||||||
 Map data Map data | Cryo-EM structure of the G protein-coupled receptor 1 (GPR1) bound to beta-arrestin 1 in ligand-free state | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | G protein-coupled receptor 1 / chemerin / beta-arrestin1 / signaling protein / MEMBRANE PROTEIN/IMMUNE SYSTEM / MEMBRANE PROTEIN-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationadipokinetic hormone binding / adipokinetic hormone receptor activity / renal water retention / Defective AVP does not bind AVPR2 and causes neurohypophyseal diabetes insipidus (NDI) / Vasopressin-like receptors / regulation of systemic arterial blood pressure by vasopressin / vasopressin receptor activity / angiotensin receptor binding / TGFBR3 regulates TGF-beta signaling / hemostasis ...adipokinetic hormone binding / adipokinetic hormone receptor activity / renal water retention / Defective AVP does not bind AVPR2 and causes neurohypophyseal diabetes insipidus (NDI) / Vasopressin-like receptors / regulation of systemic arterial blood pressure by vasopressin / vasopressin receptor activity / angiotensin receptor binding / TGFBR3 regulates TGF-beta signaling / hemostasis / Activation of SMO / telencephalon development / negative regulation of interleukin-8 production / arrestin family protein binding / G protein-coupled receptor internalization / neuropeptide binding / Lysosome Vesicle Biogenesis / : / stress fiber assembly / positive regulation of cardiac muscle hypertrophy / Golgi Associated Vesicle Biogenesis / positive regulation of Rho protein signal transduction / pseudopodium / positive regulation of systemic arterial blood pressure / negative regulation of interleukin-6 production / positive regulation of intracellular signal transduction / positive regulation of receptor internalization / positive regulation of vasoconstriction / negative regulation of Notch signaling pathway / endocytic vesicle / neuropeptide signaling pathway / activation of adenylate cyclase activity / cellular response to hormone stimulus / insulin-like growth factor receptor binding / response to cytokine / clathrin-coated pit / negative regulation of protein ubiquitination / enzyme inhibitor activity / GTPase activator activity / Activated NOTCH1 Transmits Signal to the Nucleus / cytoplasmic vesicle membrane / clathrin-coated endocytic vesicle membrane / Signaling by high-kinase activity BRAF mutants / MAP2K and MAPK activation / G protein-coupled receptor binding / G protein-coupled receptor activity / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / positive regulation of protein phosphorylation / Signaling by RAF1 mutants / Signaling by moderate kinase activity BRAF mutants / Paradoxical activation of RAF signaling by kinase inactive BRAF / Signaling downstream of RAS mutants / endocytic vesicle membrane / Signaling by BRAF and RAF1 fusions / Vasopressin regulates renal water homeostasis via Aquaporins / Cargo recognition for clathrin-mediated endocytosis / glucose homeostasis / protein transport / Clathrin-mediated endocytosis / Thrombin signalling through proteinase activated receptors (PARs) / cytoplasmic vesicle / G alpha (s) signalling events / ubiquitin-dependent protein catabolic process / molecular adaptor activity / proteasome-mediated ubiquitin-dependent protein catabolic process / transcription coactivator activity / positive regulation of ERK1 and ERK2 cascade / endosome / neuron projection / Ub-specific processing proteases / nuclear body / protein ubiquitination / G protein-coupled receptor signaling pathway / Golgi membrane / negative regulation of cell population proliferation / lysosomal membrane / positive regulation of cell population proliferation / ubiquitin protein ligase binding / positive regulation of gene expression / regulation of transcription by RNA polymerase II / chromatin / perinuclear region of cytoplasm / endoplasmic reticulum / Golgi apparatus / signal transduction / positive regulation of transcription by RNA polymerase II / nucleoplasm / membrane / nucleus / plasma membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  Escherichia phage EcSzw-2 (virus) Escherichia phage EcSzw-2 (virus) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Cai H / Lin X / Zhao L / He M / Yu J / Zhang B / Ma Y / Xie C / Shui W / Zhao Q ...Cai H / Lin X / Zhao L / He M / Yu J / Zhang B / Ma Y / Xie C / Shui W / Zhao Q / Zhu Y / Wu B | |||||||||
| Funding support |  China, 2 items China, 2 items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2025 Journal: Science / Year: 2025Title: Noncanonical agonist-dependent and -independent arrestin recruitment of GPR1. Authors: Heng Cai / Xiaowen Lin / Lechen Zhao / Maozhou He / Jie Yu / Bingjie Zhang / Yuandi Ma / Xiaohua Chang / Yuxuan Tang / Tianyu Luo / Jie Jiang / Mengna Ma / Wenqi Song / Limin Ma / Xiaojing ...Authors: Heng Cai / Xiaowen Lin / Lechen Zhao / Maozhou He / Jie Yu / Bingjie Zhang / Yuandi Ma / Xiaohua Chang / Yuxuan Tang / Tianyu Luo / Jie Jiang / Mengna Ma / Wenqi Song / Limin Ma / Xiaojing Chu / Cuiying Yi / Kun Chen / Shuo Han / Cen Xie / Wenqing Shui / Qiang Zhao / Ya Zhu / Beili Wu /  Abstract: G protein (heterotrimeric guanine nucleotide-binding protein)-coupled receptors have diverse signaling properties with differential preferences for downstream pathways. Certain receptors, such as the ...G protein (heterotrimeric guanine nucleotide-binding protein)-coupled receptors have diverse signaling properties with differential preferences for downstream pathways. Certain receptors, such as the chemerin receptor GPR1, undergo arrestin-mediated internalization but weak G protein signaling. However, the mechanisms of this unusual signaling pattern and its physiological relevance are unclear. We report the structures of GPR1 bound to chemerin and β-arrestin 1 or β-arrestin 2 and an agonist-free GPR1-β-arrestin 1 complex. Upon agonist stimulation, the receptor binds the two arrestins in distinct interaction patterns, which may account for their differential cellular responses. Agonist-independent internalization was mediated by an inactive, constitutively phosphorylated GPR1 that accommodates β-arrestin 1 in an unconventional pocket together with a fatty acid, which potentially provides a basis for GPR1 modulating lipid accumulation in lipid-overloaded adipocytes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_64619.map.gz emd_64619.map.gz | 47.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-64619-v30.xml emd-64619-v30.xml emd-64619.xml emd-64619.xml | 21.5 KB 21.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_64619.png emd_64619.png | 155.7 KB | ||
| Filedesc metadata |  emd-64619.cif.gz emd-64619.cif.gz | 7.1 KB | ||
| Others |  emd_64619_half_map_1.map.gz emd_64619_half_map_1.map.gz emd_64619_half_map_2.map.gz emd_64619_half_map_2.map.gz | 47 MB 46.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-64619 http://ftp.pdbj.org/pub/emdb/structures/EMD-64619 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-64619 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-64619 | HTTPS FTP |
-Related structure data
| Related structure data |  9uynMC  9uyhC  9uyiC  9uyjC  9uylC  9uymC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_64619.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_64619.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of the G protein-coupled receptor 1 (GPR1) bound to beta-arrestin 1 in ligand-free state | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.071 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_64619_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_64619_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : G protein-coupled receptor 1 in complex with beta-arrestin1
| Entire | Name: G protein-coupled receptor 1 in complex with beta-arrestin1 |
|---|---|
| Components |
|
-Supramolecule #1: G protein-coupled receptor 1 in complex with beta-arrestin1
| Supramolecule | Name: G protein-coupled receptor 1 in complex with beta-arrestin1 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Chemerin-like receptor 2,Vasopressin V2 receptor
| Macromolecule | Name: Chemerin-like receptor 2,Vasopressin V2 receptor / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 48.035852 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKTIIALSYI FCLVFAGSWS HPQFEKGSGA GASAGSWSHP QFEKGSDYKD DDDKEFLEVL FQGPEDLEET LFEEFENYSY DLDYYSLES DLEEKVQLGV VHWVSLVLYC LAFVLGIPGN AIVIWFTGFK WKKTVTTLWF LNLAIADFIF LLFLPLYISY V AMNFHWPF ...String: MKTIIALSYI FCLVFAGSWS HPQFEKGSGA GASAGSWSHP QFEKGSDYKD DDDKEFLEVL FQGPEDLEET LFEEFENYSY DLDYYSLES DLEEKVQLGV VHWVSLVLYC LAFVLGIPGN AIVIWFTGFK WKKTVTTLWF LNLAIADFIF LLFLPLYISY V AMNFHWPF GIWLCKANSF TAQLNMFASV FFLTVISLDH YIHLIHPCLS HRHRTLKNSL IVIIFIWLLA SLIGGPALYF RD TVEFNNH TLCYNNFQKH DPDLTLIRHH VLTWVKFIIG YLFPLLTMSI CYLCLIFKVK KRSILISSRH FWTILVVVVA FVV CWTPYH LFSIWELTIH HNSYSHHVMQ AGIPLSTGLA FLNSCLNPIL YVLISKKFQA RFRSSVAEIA RGRTPPSLGP QDE (SEP)C(TPO)(TPO)A(SEP) (SEP)(SEP)LAKDTSS UniProtKB: Chemerin-like receptor 2, Vasopressin V2 receptor |
-Macromolecule #2: Beta-arrestin-1
| Macromolecule | Name: Beta-arrestin-1 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 42.107105 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGDKGTRVFK KASPNGKLTV YLGKRDFVDH IDLVDPVDGV VLVDPEYLKE RRVYVTLTAA FRYGREDLDV LGLTFRKDLF VANVQSFPP APEDKKPLTR LQERLIKKLG EHAYPFTFEI PPNLPSSVTL QPGPEDTGKA IGVDYEVKAF VAENLEEKIH K RNSVRLVI ...String: MGDKGTRVFK KASPNGKLTV YLGKRDFVDH IDLVDPVDGV VLVDPEYLKE RRVYVTLTAA FRYGREDLDV LGLTFRKDLF VANVQSFPP APEDKKPLTR LQERLIKKLG EHAYPFTFEI PPNLPSSVTL QPGPEDTGKA IGVDYEVKAF VAENLEEKIH K RNSVRLVI EKVQYAPERP GPQPTAETTR QFLMSDKPLH LEASLDKEIY YHGEPISVNV HVTNNTNKTV KKIKISVRQY AD IVLFNTA QCKVPVAMEE ADDTVAPSST FSKVYTLTPF LANNREKRGL ALDGKLKHED TNLASSTLLR EGANREILGI IVS YKVKVK LVVSRGGLLG DLASSDVAVE LPFTLMHPKP KEEPPHREVP ENETPVDTNL UniProtKB: Beta-arrestin-1 |
-Macromolecule #3: Nanobody 32
| Macromolecule | Name: Nanobody 32 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Escherichia phage EcSzw-2 (virus) Escherichia phage EcSzw-2 (virus) |
| Molecular weight | Theoretical: 13.238716 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: QVQLQESGGG LVQAGGSLRL SCVVSGFFFD TVTMAWYRRA PGKHRELVAS ATAGGTTTYA DSVKDRFTIS RDNAKNTVYL QMNSLKPED TAVYYCNTFV RSLSWGQGTQ VTVSSHHHHH H |
-Macromolecule #4: Single-chain fragment variable 30 (scFv30)
| Macromolecule | Name: Single-chain fragment variable 30 (scFv30) / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 26.157861 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SDIQMTQSPS SLSASVGDRV TITCRASQSV SSAVAWYQQK PGKAPKLLIY SASSLYSGVP SRFSGSRSGT DFTLTISSLQ PEDFATYYC QQYKYVPVTF GQGTKVEIKG TTAASGSSGG SSSGAEVQLV ESGGGLVQPG GSLRLSCAAS GFNVYSSSIH W VRQAPGKG ...String: SDIQMTQSPS SLSASVGDRV TITCRASQSV SSAVAWYQQK PGKAPKLLIY SASSLYSGVP SRFSGSRSGT DFTLTISSLQ PEDFATYYC QQYKYVPVTF GQGTKVEIKG TTAASGSSGG SSSGAEVQLV ESGGGLVQPG GSLRLSCAAS GFNVYSSSIH W VRQAPGKG LEWVASISSY YGYTYYADSV KGRFTISADT SKNTAYLQMN SLRAEDTAVY YCARSRQFWY SGLDYWGQGT LV TVSSA |
-Macromolecule #5: PALMITOLEIC ACID
| Macromolecule | Name: PALMITOLEIC ACID / type: ligand / ID: 5 / Number of copies: 1 / Formula: PAM |
|---|---|
| Molecular weight | Theoretical: 254.408 Da |
| Chemical component information | 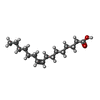 ChemComp-PAM: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 2.1875 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




































