[English] 日本語
 Yorodumi
Yorodumi- PDB-9uyn: Cryo-EM structure of the G protein-coupled receptor 1 (GPR1) boun... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 9uyn | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the G protein-coupled receptor 1 (GPR1) bound to beta-arrestin 1 in ligand-free state | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | MEMBRANE PROTEIN/IMMUNE SYSTEM / G protein-coupled receptor 1 / chemerin / beta-arrestin1 / signaling protein / MEMBRANE PROTEIN-IMMUNE SYSTEM complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationadipokinetic hormone binding / adipokinetic hormone receptor activity / renal water retention / Defective AVP does not bind AVPR2 and causes neurohypophyseal diabetes insipidus (NDI) / Vasopressin-like receptors / regulation of systemic arterial blood pressure by vasopressin / vasopressin receptor activity / angiotensin receptor binding / TGFBR3 regulates TGF-beta signaling / hemostasis ...adipokinetic hormone binding / adipokinetic hormone receptor activity / renal water retention / Defective AVP does not bind AVPR2 and causes neurohypophyseal diabetes insipidus (NDI) / Vasopressin-like receptors / regulation of systemic arterial blood pressure by vasopressin / vasopressin receptor activity / angiotensin receptor binding / TGFBR3 regulates TGF-beta signaling / hemostasis / Activation of SMO / telencephalon development / negative regulation of interleukin-8 production / arrestin family protein binding / G protein-coupled receptor internalization / neuropeptide binding / Lysosome Vesicle Biogenesis / : / positive regulation of cardiac muscle hypertrophy / stress fiber assembly / Golgi Associated Vesicle Biogenesis / positive regulation of Rho protein signal transduction / positive regulation of vasoconstriction / pseudopodium / positive regulation of systemic arterial blood pressure / negative regulation of interleukin-6 production / positive regulation of intracellular signal transduction / positive regulation of receptor internalization / negative regulation of Notch signaling pathway / endocytic vesicle / enzyme inhibitor activity / neuropeptide signaling pathway / activation of adenylate cyclase activity / cellular response to hormone stimulus / insulin-like growth factor receptor binding / response to cytokine / clathrin-coated pit / negative regulation of protein ubiquitination / GTPase activator activity / Activated NOTCH1 Transmits Signal to the Nucleus / cytoplasmic vesicle membrane / clathrin-coated endocytic vesicle membrane / Signaling by high-kinase activity BRAF mutants / MAP2K and MAPK activation / G protein-coupled receptor binding / G protein-coupled receptor activity / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / positive regulation of protein phosphorylation / Signaling by RAF1 mutants / Signaling by moderate kinase activity BRAF mutants / Paradoxical activation of RAF signaling by kinase inactive BRAF / Signaling downstream of RAS mutants / endocytic vesicle membrane / Signaling by BRAF and RAF1 fusions / Vasopressin regulates renal water homeostasis via Aquaporins / Cargo recognition for clathrin-mediated endocytosis / glucose homeostasis / protein transport / Clathrin-mediated endocytosis / Thrombin signalling through proteinase activated receptors (PARs) / cytoplasmic vesicle / ubiquitin-dependent protein catabolic process / G alpha (s) signalling events / molecular adaptor activity / proteasome-mediated ubiquitin-dependent protein catabolic process / transcription coactivator activity / positive regulation of ERK1 and ERK2 cascade / endosome / neuron projection / Ub-specific processing proteases / nuclear body / protein ubiquitination / G protein-coupled receptor signaling pathway / Golgi membrane / negative regulation of cell population proliferation / lysosomal membrane / intracellular membrane-bounded organelle / positive regulation of cell population proliferation / ubiquitin protein ligase binding / positive regulation of gene expression / regulation of transcription by RNA polymerase II / chromatin / perinuclear region of cytoplasm / endoplasmic reticulum / Golgi apparatus / signal transduction / positive regulation of transcription by RNA polymerase II / nucleoplasm / nucleus / membrane / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) Escherichia phage EcSzw-2 (virus) Escherichia phage EcSzw-2 (virus) | |||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Cai, H. / Lin, X. / Zhao, L. / He, M. / Yu, J. / Zhang, B. / Ma, Y. / Xie, C. / Shui, W. / Zhao, Q. ...Cai, H. / Lin, X. / Zhao, L. / He, M. / Yu, J. / Zhang, B. / Ma, Y. / Xie, C. / Shui, W. / Zhao, Q. / Zhu, Y. / Wu, B. | |||||||||
| Funding support |  China, 2items China, 2items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2025 Journal: Science / Year: 2025Title: Noncanonical agonist-dependent and -independent arrestin recruitment of GPR1. Authors: Heng Cai / Xiaowen Lin / Lechen Zhao / Maozhou He / Jie Yu / Bingjie Zhang / Yuandi Ma / Xiaohua Chang / Yuxuan Tang / Tianyu Luo / Jie Jiang / Mengna Ma / Wenqi Song / Limin Ma / Xiaojing ...Authors: Heng Cai / Xiaowen Lin / Lechen Zhao / Maozhou He / Jie Yu / Bingjie Zhang / Yuandi Ma / Xiaohua Chang / Yuxuan Tang / Tianyu Luo / Jie Jiang / Mengna Ma / Wenqi Song / Limin Ma / Xiaojing Chu / Cuiying Yi / Kun Chen / Shuo Han / Cen Xie / Wenqing Shui / Qiang Zhao / Ya Zhu / Beili Wu /  Abstract: G protein (heterotrimeric guanine nucleotide-binding protein)-coupled receptors have diverse signaling properties with differential preferences for downstream pathways. Certain receptors, such as the ...G protein (heterotrimeric guanine nucleotide-binding protein)-coupled receptors have diverse signaling properties with differential preferences for downstream pathways. Certain receptors, such as the chemerin receptor GPR1, undergo arrestin-mediated internalization but weak G protein signaling. However, the mechanisms of this unusual signaling pattern and its physiological relevance are unclear. We report the structures of GPR1 bound to chemerin and β-arrestin 1 or β-arrestin 2 and an agonist-free GPR1-β-arrestin 1 complex. Upon agonist stimulation, the receptor binds the two arrestins in distinct interaction patterns, which may account for their differential cellular responses. Agonist-independent internalization was mediated by an inactive, constitutively phosphorylated GPR1 that accommodates β-arrestin 1 in an unconventional pocket together with a fatty acid, which potentially provides a basis for GPR1 modulating lipid accumulation in lipid-overloaded adipocytes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  9uyn.cif.gz 9uyn.cif.gz | 188.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb9uyn.ent.gz pdb9uyn.ent.gz | 141.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  9uyn.json.gz 9uyn.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  9uyn_validation.pdf.gz 9uyn_validation.pdf.gz | 1.1 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  9uyn_full_validation.pdf.gz 9uyn_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  9uyn_validation.xml.gz 9uyn_validation.xml.gz | 37.9 KB | Display | |
| Data in CIF |  9uyn_validation.cif.gz 9uyn_validation.cif.gz | 55.3 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/uy/9uyn https://data.pdbj.org/pub/pdb/validation_reports/uy/9uyn ftp://data.pdbj.org/pub/pdb/validation_reports/uy/9uyn ftp://data.pdbj.org/pub/pdb/validation_reports/uy/9uyn | HTTPS FTP |
-Related structure data
| Related structure data |  64619MC  9uyhC  9uyiC  9uyjC  9uylC  9uymC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 48035.852 Da / Num. of mol.: 1 / Mutation: V143C Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CMKLR2, GPR1, AVPR2, ADHR, DIR, DIR3, V2R / Production host: Homo sapiens (human) / Gene: CMKLR2, GPR1, AVPR2, ADHR, DIR, DIR3, V2R / Production host:  |
|---|---|
| #2: Protein | Mass: 42107.105 Da / Num. of mol.: 1 Mutation: C59A/C125S/C140I/C150V/R169E/C242V/Y249C/C251V/C269S Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: ARRB1, ARR1 / Production host: Homo sapiens (human) / Gene: ARRB1, ARR1 / Production host:  |
| #3: Antibody | Mass: 13238.716 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Escherichia phage EcSzw-2 (virus) / Production host: Escherichia phage EcSzw-2 (virus) / Production host:  |
| #4: Antibody | Mass: 26157.861 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:  |
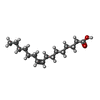
| #5: Chemical | ChemComp-PAM / |
| Has ligand of interest | Y |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: G protein-coupled receptor 1 in complex with beta-arrestin1 Type: COMPLEX / Entity ID: #1-#4 / Source: MULTIPLE SOURCES |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  |
| Buffer solution | pH: 7.5 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: TFS KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN |
| Electron lens | Mode: BRIGHT FIELD / Nominal defocus max: 1500 nm / Nominal defocus min: 800 nm |
| Image recording | Electron dose: 2.1875 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) |
- Processing
Processing
| EM software | Name: PHENIX / Version: 1.18.2_3874 / Category: model refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Type: NONE | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 2.9 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 413367 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refinement | Stereochemistry target values: REAL-SPACE (WEIGHTED MAP SUM AT ATOM CENTERS) | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller










 PDBj
PDBj