+Search query
-Structure paper
| Title | Structural analysis of PSI-ACPI and PSII-ACPII supercomplexes from a cryptophyte alga sp. NIES-2332. |
|---|---|
| Journal, issue, pages | Front Plant Sci, Vol. 16, Page 1716939, Year 2025 |
| Publish date | Nov 27, 2025 |
 Authors Authors | Wenyue Zhang / Nozomi Yonehara / Mizuki Ishii / Haowei Jiang / Romain La Rocca / Pi-Cheng Tsai / Hongjie Li / Koji Kato / Fusamichi Akita / Jian-Ren Shen |
| PubMed Abstract | Light energy is converted to chemical energy by two photosystems (PSI and PSII) in complex with their light-harvesting complex proteins (LHCI and LHCII) in photosynthesis. is a member of cryptophyte ...Light energy is converted to chemical energy by two photosystems (PSI and PSII) in complex with their light-harvesting complex proteins (LHCI and LHCII) in photosynthesis. is a member of cryptophyte alga whose LHCs contain unique chlorophyll proteins (ACPs) and phycobiliproteins. We purified PSI-ACPI and PSII-ACPII supercomplexes from a cryptophyte sp. NIES-2332 and analyzed their structures at high resolutions of 2.08 Å and 2.17 Å, respectively, using cryo-electron microscopy. These structures are largely similar to those reported previously from two other species of cryptophytes, but exhibited some differences in both the pigment locations and subunit structures. A part of the antenna subunits of both photosystems is shifted compared with the previously reported structures from other species of cryptophytes, suggesting some differences in the energy transfer rates from the antenna to the PSI and PSII cores. Newly identified lipids are found to occupy the interfaces between the antennae and cores, which may be important for assembly and stabilization of the supercomplexes. Water molecules surrounding three iron-sulfur clusters of the PSI core are found in our high-resolution structure, some of which are conserved from cyanobacteria to higher plants but some are different. In addition, our structure of PSII-ACPII lacks the subunits of oxygen-evolving complex as well as the MnCaO cluster, suggesting that the cells are in the S-growth phase, yet the PSI-ACPI structure showed the binding of PsaQ, suggesting that it is in an L-phase. These results suggest that the S-phase and L-phase can co-exist in the cryptophytic cells. The high-resolution structures of both PSI-ACPIs and PSII-ACPIIs solved in this study provide a more solid structural basis for elucidating the energy transfer and quenching mechanisms in this group of the organisms. |
 External links External links |  Front Plant Sci / Front Plant Sci /  PubMed:41393888 / PubMed:41393888 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 2.08 - 2.17 Å |
| Structure data | EMDB-62656, PDB-9kz9: EMDB-62717, PDB-9l0k: EMDB-62846, PDB-9l5v: |
| Chemicals | 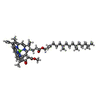 ChemComp-CL0:  ChemComp-CLA: 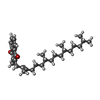 ChemComp-PQN:  ChemComp-LHG: 
ChemComp-WVN: 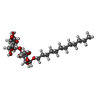 ChemComp-LMU:  ChemComp-SF4:  ChemComp-SQD: 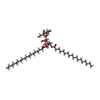 ChemComp-DGD:  ChemComp-LMG:  ChemComp-II0:  ChemComp-IHT:  ChemComp-KC2:  ChemComp-HOH:  ChemComp-FE2:  ChemComp-PHO:  ChemComp-PL9:  ChemComp-CL:  ChemComp-MN:  ChemComp-BCT:  ChemComp-HEM: |
| Source |
|
 Keywords Keywords | PHOTOSYNTHESIS / photosynthesis complex |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers









 rhodomonas sp. nies-2332 (eukaryote)
rhodomonas sp. nies-2332 (eukaryote)