+Search query
-Structure paper
| Title | Structural basis of the acyl-transfer mechanism of human GPAT1. |
|---|---|
| Journal, issue, pages | Nat Struct Mol Biol, Vol. 30, Issue 1, Page 22-30, Year 2023 |
| Publish date | Dec 15, 2022 |
 Authors Authors | Zachary Lee Johnson / Mark Ammirati / David Jonathan Wasilko / Jeanne S Chang / Stephen Noell / Timothy L Foley / Hyejin Yoon / Kathleen Smith / Shoh Asano / Katherine Hales / Min Wan / Qingyi Yang / Mary A Piotrowski / Kathleen A Farley / Tamara Gilbert / Lisa M Aschenbrenner / Kimberly F Fennell / Jason K Dutra / Mary Xu / Chunyang Guo / Alison E Varghese / Justin Bellenger / Alandra Quinn / Christopher W Am Ende / Graham M West / Matthew C Griffor / Donald Bennett / Matthew Calabrese / Claire M Steppan / Seungil Han / Huixian Wu /  |
| PubMed Abstract | Glycerol-3-phosphate acyltransferase (GPAT)1 is a mitochondrial outer membrane protein that catalyzes the first step of de novo glycerolipid biosynthesis. Hepatic expression of GPAT1 is linked to ...Glycerol-3-phosphate acyltransferase (GPAT)1 is a mitochondrial outer membrane protein that catalyzes the first step of de novo glycerolipid biosynthesis. Hepatic expression of GPAT1 is linked to liver fat accumulation and the severity of nonalcoholic fatty liver diseases. Here we present the cryo-EM structures of human GPAT1 in substrate analog-bound and product-bound states. The structures reveal an N-terminal acyltransferase domain that harbors important catalytic motifs and a tightly associated C-terminal domain that is critical for proper protein folding. Unexpectedly, GPAT1 has no transmembrane regions as previously proposed but instead associates with the membrane via an amphipathic surface patch and an N-terminal loop-helix region that contains a mitochondrial-targeting signal. Combined structural, computational and functional studies uncover a hydrophobic pathway within GPAT1 for lipid trafficking. The results presented herein lay a framework for rational inhibitor development for GPAT1. |
 External links External links |  Nat Struct Mol Biol / Nat Struct Mol Biol /  PubMed:36522428 PubMed:36522428 |
| Methods | EM (single particle) |
| Resolution | 3.4 - 3.67 Å |
| Structure data | EMDB-27898, PDB-8e4y: EMDB-27899, PDB-8e50: |
| Chemicals | 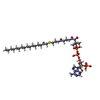 ChemComp-UKL: 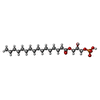 ChemComp-NKO:  ChemComp-COA: |
| Source |
|
 Keywords Keywords | MEMBRANE PROTEIN / acyltransferase / LPA / monotopic / mitochondrial |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers







 homo sapiens (human)
homo sapiens (human)