[English] 日本語
 Yorodumi
Yorodumi- EMDB-27898: Cryo-EM structure of human glycerol-3-phosphate acyltransferase 1... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
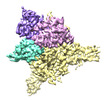
| Title | Cryo-EM structure of human glycerol-3-phosphate acyltransferase 1 (GPAT1) in complex with 2-oxohexadecyl-CoA | |||||||||
 Map data Map data | Sharpened Full Map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | acyltransferase / LPA / monotopic / mitochondrial / membrane protein | |||||||||
| Function / homology |  Function and homology information Function and homology informationglycerol-3-phosphate 1-O-acyltransferase / glycerol-3-phosphate O-acyltransferase activity / phosphatidylglycerol biosynthetic process / CDP-diacylglycerol biosynthetic process / Triglyceride biosynthesis / diacylglycerol biosynthetic process / glycerophospholipid metabolic process / negative regulation of activation-induced cell death of T cells / triglyceride biosynthetic process / phosphatidic acid biosynthetic process ...glycerol-3-phosphate 1-O-acyltransferase / glycerol-3-phosphate O-acyltransferase activity / phosphatidylglycerol biosynthetic process / CDP-diacylglycerol biosynthetic process / Triglyceride biosynthesis / diacylglycerol biosynthetic process / glycerophospholipid metabolic process / negative regulation of activation-induced cell death of T cells / triglyceride biosynthetic process / phosphatidic acid biosynthetic process / Synthesis of PA / activation-induced cell death of T cells / acyl-CoA metabolic process / phospholipid homeostasis / activated T cell proliferation / positive regulation of multicellular organism growth / RUNX1 regulates estrogen receptor mediated transcription / positive regulation of activated T cell proliferation / fatty acid homeostasis / response to glucose / regulation of cytokine production / Activation of gene expression by SREBF (SREBP) / fatty acid metabolic process / MLL4 and MLL3 complexes regulate expression of PPARG target genes in adipogenesis and hepatic steatosis / Estrogen-dependent gene expression / defense response to virus / mitochondrial outer membrane / mitochondrion / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
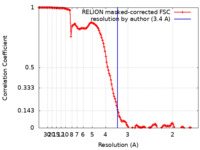
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Johnson ZL / Wasilko DJ / Ammirati M / Chang JS / Han S / Wu H | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2023 Journal: Nat Struct Mol Biol / Year: 2023Title: Structural basis of the acyl-transfer mechanism of human GPAT1. Authors: Zachary Lee Johnson / Mark Ammirati / David Jonathan Wasilko / Jeanne S Chang / Stephen Noell / Timothy L Foley / Hyejin Yoon / Kathleen Smith / Shoh Asano / Katherine Hales / Min Wan / ...Authors: Zachary Lee Johnson / Mark Ammirati / David Jonathan Wasilko / Jeanne S Chang / Stephen Noell / Timothy L Foley / Hyejin Yoon / Kathleen Smith / Shoh Asano / Katherine Hales / Min Wan / Qingyi Yang / Mary A Piotrowski / Kathleen A Farley / Tamara Gilbert / Lisa M Aschenbrenner / Kimberly F Fennell / Jason K Dutra / Mary Xu / Chunyang Guo / Alison E Varghese / Justin Bellenger / Alandra Quinn / Christopher W Am Ende / Graham M West / Matthew C Griffor / Donald Bennett / Matthew Calabrese / Claire M Steppan / Seungil Han / Huixian Wu /  Abstract: Glycerol-3-phosphate acyltransferase (GPAT)1 is a mitochondrial outer membrane protein that catalyzes the first step of de novo glycerolipid biosynthesis. Hepatic expression of GPAT1 is linked to ...Glycerol-3-phosphate acyltransferase (GPAT)1 is a mitochondrial outer membrane protein that catalyzes the first step of de novo glycerolipid biosynthesis. Hepatic expression of GPAT1 is linked to liver fat accumulation and the severity of nonalcoholic fatty liver diseases. Here we present the cryo-EM structures of human GPAT1 in substrate analog-bound and product-bound states. The structures reveal an N-terminal acyltransferase domain that harbors important catalytic motifs and a tightly associated C-terminal domain that is critical for proper protein folding. Unexpectedly, GPAT1 has no transmembrane regions as previously proposed but instead associates with the membrane via an amphipathic surface patch and an N-terminal loop-helix region that contains a mitochondrial-targeting signal. Combined structural, computational and functional studies uncover a hydrophobic pathway within GPAT1 for lipid trafficking. The results presented herein lay a framework for rational inhibitor development for GPAT1. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27898.map.gz emd_27898.map.gz | 202.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27898-v30.xml emd-27898-v30.xml emd-27898.xml emd-27898.xml | 21.2 KB 21.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_27898_fsc.xml emd_27898_fsc.xml | 13.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_27898.png emd_27898.png | 133.7 KB | ||
| Filedesc metadata |  emd-27898.cif.gz emd-27898.cif.gz | 7.1 KB | ||
| Others |  emd_27898_half_map_1.map.gz emd_27898_half_map_1.map.gz emd_27898_half_map_2.map.gz emd_27898_half_map_2.map.gz | 171.1 MB 170.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27898 http://ftp.pdbj.org/pub/emdb/structures/EMD-27898 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27898 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27898 | HTTPS FTP |
-Related structure data
| Related structure data |  8e4yMC  8e50C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27898.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27898.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Sharpened Full Map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.84 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half Map
| File | emd_27898_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half Map
| File | emd_27898_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : glycerol-3-phosphate acyltransferase 1
| Entire | Name: glycerol-3-phosphate acyltransferase 1 |
|---|---|
| Components |
|
-Supramolecule #1: glycerol-3-phosphate acyltransferase 1
| Supramolecule | Name: glycerol-3-phosphate acyltransferase 1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 86 KDa |
-Macromolecule #1: Glycerol-3-phosphate acyltransferase 1, mitochondrial
| Macromolecule | Name: Glycerol-3-phosphate acyltransferase 1, mitochondrial / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: glycerol-3-phosphate 1-O-acyltransferase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 86.919469 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDYKDDDDKG SENLYFQSNP SIPSLGLRNV IYINETHTRH RGWLARRLSY VLFIQERDVH KGMFATNVTE NVLNSSRVQE AIAEVAAEL NPDGSAQQQS KAVNKVKKKA KRILQEMVAT VSPAMIRLTG WVLLKLFNSF FWNIQIHKGQ LEMVKAATET N LPLLFLPV ...String: MDYKDDDDKG SENLYFQSNP SIPSLGLRNV IYINETHTRH RGWLARRLSY VLFIQERDVH KGMFATNVTE NVLNSSRVQE AIAEVAAEL NPDGSAQQQS KAVNKVKKKA KRILQEMVAT VSPAMIRLTG WVLLKLFNSF FWNIQIHKGQ LEMVKAATET N LPLLFLPV HRSHIDYLLL TFILFCHNIK APYIASGNNL NIPIFSTLIH KLGGFFIRRR LDETPDGRKD VLYRALLHGH IV ELLRQQQ FLEIFLEGTR SRSGKTSCAR AGLLSVVVDT LSTNVIPDIL IIPVGISYDR IIEGHYNGEQ LGKPKKNESL WSV ARGVIR MLRKNYGCVR VDFAQPFSLK EYLESQSQKP VSALLSLEQA LLPAILPSRP SDAADEGRDT SINESRNATD ESLR RRLIA NLAEHILFTA SKSCAIMSTH IVACLLLYRH RQGIDLSTLV EDFFVMKEEV LARDFDLGFS GNSEDVVMHA IQLLG NCVT ITHTSRNDEF FITPSTTVPS VFELNFYSNG VLHVFIMEAI IACSLYAVLN KRGLGGPTST PPNLISQEQL VRKAAS LCY LLSNEGTISL PCQTFYQVCH ETVGKFIQYG ILTVAEHDDQ EDISPSLAEQ QWDKKLPEPL SWRSDEEDED SDFGEEQ RD CYLKVSQSKE HQQFITFLQR LLGPLLEAYS SAAIFVHNFS GPVPEPEYLQ KLHKYLITRT ERNVAVYAES ATYCLVKN A VKMFKDIGVF KETKQKRVSV LELSSTFLPQ CNRQKLLEYI LSFVVL UniProtKB: Glycerol-3-phosphate acyltransferase 1, mitochondrial |
-Macromolecule #2: [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonoox...
| Macromolecule | Name: [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methyl (3R)-3-hydroxy-2,2-dimethyl-4-oxo-4-{[3-oxo-3-({2-[(2-oxohexadecyl)sulfanyl]ethyl}amino)propyl]amino}butyl ...Name: [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-hydroxy-3-(phosphonooxy)oxolan-2-yl]methyl (3R)-3-hydroxy-2,2-dimethyl-4-oxo-4-{[3-oxo-3-({2-[(2-oxohexadecyl)sulfanyl]ethyl}amino)propyl]amino}butyl dihydrogen diphosphate (non-preferred name) type: ligand / ID: 2 / Number of copies: 1 / Formula: UKL |
|---|---|
| Molecular weight | Theoretical: 1.005943 KDa |
| Chemical component information | 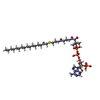 ChemComp-UKL: |
-Macromolecule #3: (2R)-2-hydroxy-3-(phosphonooxy)propyl hexadecanoate
| Macromolecule | Name: (2R)-2-hydroxy-3-(phosphonooxy)propyl hexadecanoate / type: ligand / ID: 3 / Number of copies: 1 / Formula: NKO |
|---|---|
| Molecular weight | Theoretical: 410.483 Da |
| Chemical component information | 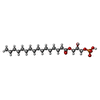 ChemComp-NKO: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5.7 mg/mL | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| ||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 40 sec. / Pretreatment - Atmosphere: OTHER | ||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: Blot force -5, blot time 3 sec. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Number grids imaged: 2 / Number real images: 8491 / Average exposure time: 7.0 sec. / Average electron dose: 74.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 0.6 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)