+Search query
-Structure paper
| Title | Enterovirus particles expel capsid pentamers to enable genome release. |
|---|---|
| Journal, issue, pages | Nat Commun, Vol. 10, Issue 1, Page 1138, Year 2019 |
| Publish date | Mar 8, 2019 |
 Authors Authors | David Buchta / Tibor Füzik / Dominik Hrebík / Yevgen Levdansky / Lukáš Sukeník / Liya Mukhamedova / Jana Moravcová / Robert Vácha / Pavel Plevka /   |
| PubMed Abstract | Viruses from the genus Enterovirus are important human pathogens. Receptor binding or exposure to acidic pH in endosomes converts enterovirus particles to an activated state that is required for ...Viruses from the genus Enterovirus are important human pathogens. Receptor binding or exposure to acidic pH in endosomes converts enterovirus particles to an activated state that is required for genome release. However, the mechanism of enterovirus uncoating is not well understood. Here, we use cryo-electron microscopy to visualize virions of human echovirus 18 in the process of genome release. We discover that the exit of the RNA from the particle of echovirus 18 results in a loss of one, two, or three adjacent capsid-protein pentamers. The opening in the capsid, which is more than 120 Å in diameter, enables the release of the genome without the need to unwind its putative double-stranded RNA segments. We also detect capsids lacking pentamers during genome release from echovirus 30. Thus, our findings uncover a mechanism of enterovirus genome release that could become target for antiviral drugs. |
 External links External links |  Nat Commun / Nat Commun /  PubMed:30850609 / PubMed:30850609 /  PubMed Central PubMed Central |
| Methods | EM (single particle) |
| Resolution | 3.16 - 19.4 Å |
| Structure data |  EMDB-0184: EMDB-0185, PDB-6hbk:  EMDB-0186:  EMDB-0187: EMDB-0188, PDB-6hbl:  EMDB-0189: |
| Chemicals |  ChemComp-PLM: 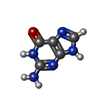 ChemComp-GUN: |
| Source |
|
 Keywords Keywords | VIRUS / echovirus / echovirus 18 / native particle / enterovirus / picornavirus / altered particle / A-particle / empty particle / B-particle / open particle / O-particle / genome release |
 Movie
Movie Controller
Controller Structure viewers
Structure viewers About Yorodumi Papers
About Yorodumi Papers















 echovirus e18
echovirus e18