[English] 日本語
 Yorodumi
Yorodumi- EMDB-7459: Cryo-EM structure at 3.8 A resolution of vaccine-elicited antibod... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-7459 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure at 3.8 A resolution of vaccine-elicited antibody vFP20.01 in complex with HIV-1 Env BG505 DS-SOSIP, and antibodies VRC03 and PGT122 | |||||||||
 Map data Map data | primary map | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / positive regulation of establishment of T cell polarity / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral protein processing / fusion of virus membrane with host plasma membrane / virus-mediated perturbation of host defense response / fusion of virus membrane with host endosome membrane / viral envelope ...positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / positive regulation of establishment of T cell polarity / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral protein processing / fusion of virus membrane with host plasma membrane / virus-mediated perturbation of host defense response / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell / apoptotic process / host cell plasma membrane / structural molecule activity / virion membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /   Human immunodeficiency virus 1 Human immunodeficiency virus 1 | |||||||||
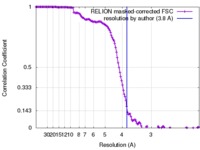
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Acharya P / Carragher B / Potter CS / Kwong PD | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Med / Year: 2018 Journal: Nat Med / Year: 2018Title: Epitope-based vaccine design yields fusion peptide-directed antibodies that neutralize diverse strains of HIV-1. Authors: Kai Xu / Priyamvada Acharya / Rui Kong / Cheng Cheng / Gwo-Yu Chuang / Kevin Liu / Mark K Louder / Sijy O'Dell / Reda Rawi / Mallika Sastry / Chen-Hsiang Shen / Baoshan Zhang / Tongqing Zhou ...Authors: Kai Xu / Priyamvada Acharya / Rui Kong / Cheng Cheng / Gwo-Yu Chuang / Kevin Liu / Mark K Louder / Sijy O'Dell / Reda Rawi / Mallika Sastry / Chen-Hsiang Shen / Baoshan Zhang / Tongqing Zhou / Mangaiarkarasi Asokan / Robert T Bailer / Michael Chambers / Xuejun Chen / Chang W Choi / Venkata P Dandey / Nicole A Doria-Rose / Aliaksandr Druz / Edward T Eng / S Katie Farney / Kathryn E Foulds / Hui Geng / Ivelin S Georgiev / Jason Gorman / Kurt R Hill / Alexander J Jafari / Young D Kwon / Yen-Ting Lai / Thomas Lemmin / Krisha McKee / Tiffany Y Ohr / Li Ou / Dongjun Peng / Ariana P Rowshan / Zizhang Sheng / John-Paul Todd / Yaroslav Tsybovsky / Elise G Viox / Yiran Wang / Hui Wei / Yongping Yang / Amy F Zhou / Rui Chen / Lu Yang / Diana G Scorpio / Adrian B McDermott / Lawrence Shapiro / Bridget Carragher / Clinton S Potter / John R Mascola / Peter D Kwong /  Abstract: A central goal of HIV-1 vaccine research is the elicitation of antibodies capable of neutralizing diverse primary isolates of HIV-1. Here we show that focusing the immune response to exposed N- ...A central goal of HIV-1 vaccine research is the elicitation of antibodies capable of neutralizing diverse primary isolates of HIV-1. Here we show that focusing the immune response to exposed N-terminal residues of the fusion peptide, a critical component of the viral entry machinery and the epitope of antibodies elicited by HIV-1 infection, through immunization with fusion peptide-coupled carriers and prefusion stabilized envelope trimers, induces cross-clade neutralizing responses. In mice, these immunogens elicited monoclonal antibodies capable of neutralizing up to 31% of a cross-clade panel of 208 HIV-1 strains. Crystal and cryoelectron microscopy structures of these antibodies revealed fusion peptide conformational diversity as a molecular explanation for the cross-clade neutralization. Immunization of guinea pigs and rhesus macaques induced similarly broad fusion peptide-directed neutralizing responses, suggesting translatability. The N terminus of the HIV-1 fusion peptide is thus a promising target of vaccine efforts aimed at eliciting broadly neutralizing antibodies. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_7459.map.gz emd_7459.map.gz | 203.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-7459-v30.xml emd-7459-v30.xml emd-7459.xml emd-7459.xml | 34.8 KB 34.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_7459_fsc.xml emd_7459_fsc.xml | 13.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_7459.png emd_7459.png | 128.9 KB | ||
| Masks |  emd_7459_msk_1.map emd_7459_msk_1.map emd_7459_msk_2.map emd_7459_msk_2.map | 216 MB 216 MB |  Mask map Mask map | |
| Others |  emd_7459_additional_1.map.gz emd_7459_additional_1.map.gz emd_7459_additional_2.map.gz emd_7459_additional_2.map.gz emd_7459_additional_3.map.gz emd_7459_additional_3.map.gz emd_7459_additional_4.map.gz emd_7459_additional_4.map.gz | 107.8 MB 20.3 MB 13.9 MB 20.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-7459 http://ftp.pdbj.org/pub/emdb/structures/EMD-7459 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7459 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7459 | HTTPS FTP |
-Validation report
| Summary document |  emd_7459_validation.pdf.gz emd_7459_validation.pdf.gz | 555.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_7459_full_validation.pdf.gz emd_7459_full_validation.pdf.gz | 555 KB | Display | |
| Data in XML |  emd_7459_validation.xml.gz emd_7459_validation.xml.gz | 13.9 KB | Display | |
| Data in CIF |  emd_7459_validation.cif.gz emd_7459_validation.cif.gz | 18.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7459 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7459 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7459 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-7459 | HTTPS FTP |
-Related structure data
| Related structure data |  6cdeMC  7460C  8420C  8421C  8422C  5tkjC  5tkkC  6cdiC  6cdmC  6cdoC  6cdpC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_7459.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_7459.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | primary map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
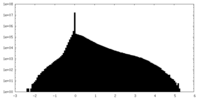
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
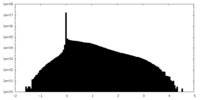
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_7459_msk_1.map emd_7459_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Mask #2
| File |  emd_7459_msk_2.map emd_7459_msk_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: additional map #1
| File | emd_7459_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | additional map #1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: additional map #2
| File | emd_7459_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | additional map #2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: additional map #3
| File | emd_7459_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | additional map #3 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: additional map #4
| File | emd_7459_additional_4.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | additional map #4 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : vFP20.01-BG505 DS-SOSIP-VRC03-PGT122
+Supramolecule #1: vFP20.01-BG505 DS-SOSIP-VRC03-PGT122
+Supramolecule #2: PGT122
+Supramolecule #3: VRC03
+Supramolecule #4: Glycoprotein
+Supramolecule #5: vFP20.01
+Macromolecule #1: vFP20.01 Heavy Chain
+Macromolecule #2: vFP20.01 Light chain
+Macromolecule #3: PGT122 Heavy chain
+Macromolecule #4: PGT122 Light Chain
+Macromolecule #5: VRC03 Light Chain
+Macromolecule #6: VRC03 Heavy Chain
+Macromolecule #7: Glycoprotein 41
+Macromolecule #8: Glycoprotein 120
+Macromolecule #18: 2-acetamido-2-deoxy-beta-D-glucopyranose
+Macromolecule #19: alpha-D-mannopyranose
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 7 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-40 / Number grids imaged: 1 / Number real images: 723 / Average electron dose: 70.7 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: -2.8 µm / Calibrated defocus min: -1.8 µm / Calibrated magnification: 130000 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 107 / Target criteria: Correlation Coefficient |
|---|---|
| Output model |  PDB-6cde: |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)