+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-5724 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Bacteriophage Sf6 procapsid | |||||||||
 Map data Map data | sf6 procapsid icosahedral reconstruction | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | icosahedral reconstruction of bacteriophage Sf6 procapsids | |||||||||
| Biological species |  Shigella phage Sf6 (virus) Shigella phage Sf6 (virus) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 7.0 Å | |||||||||
 Authors Authors | Parent KN / Gilcrease EB / Casjens SR / Baker TS / Tang J | |||||||||
 Citation Citation |  Journal: Virology / Year: 2012 Journal: Virology / Year: 2012Title: Structural evolution of the P22-like phages: comparison of Sf6 and P22 procapsid and virion architectures. Authors: Kristin N Parent / Eddie B Gilcrease / Sherwood R Casjens / Timothy S Baker /  Abstract: Coat proteins of tailed, dsDNA phages and in herpesviruses include a conserved core similar to the bacteriophage HK97 subunit. This core is often embellished with other domains such as the telokin Ig- ...Coat proteins of tailed, dsDNA phages and in herpesviruses include a conserved core similar to the bacteriophage HK97 subunit. This core is often embellished with other domains such as the telokin Ig-like domain of phage P22. Eighty-six P22-like phages and prophages with sequenced genomes share a similar set of virion assembly genes and, based on comparisons of twelve viral assembly proteins (structural and assembly/packaging chaperones), these phages are classified into three groups (P22-like, Sf6-like, and CUS-3-like). We used cryo-electron microscopy and 3D image reconstruction to determine the structures of Sf6 procapsids and virions (~7Å resolution), and the structure of the entire, asymmetric Sf6 virion (16-Å resolution). The Sf6 coat protein is similar to that of P22 yet it has differences in the telokin domain and in its overall quaternary organization. Thermal stability and agarose gel experiments show that Sf6 virions are slightly less stable than those of P22. Finally, bacterial host outer membrane proteins A and C were identified in lipid vesicles that co-purify with Sf6 particles, but are not components of the capsid. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_5724.map.gz emd_5724.map.gz | 623 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-5724-v30.xml emd-5724-v30.xml emd-5724.xml emd-5724.xml | 9.6 KB 9.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_5724_1.png emd_5724_1.png | 2.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-5724 http://ftp.pdbj.org/pub/emdb/structures/EMD-5724 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5724 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-5724 | HTTPS FTP |
-Validation report
| Summary document |  emd_5724_validation.pdf.gz emd_5724_validation.pdf.gz | 78.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_5724_full_validation.pdf.gz emd_5724_full_validation.pdf.gz | 77.2 KB | Display | |
| Data in XML |  emd_5724_validation.xml.gz emd_5724_validation.xml.gz | 493 B | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5724 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5724 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5724 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-5724 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_5724.map.gz / Format: CCP4 / Size: 1.6 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_5724.map.gz / Format: CCP4 / Size: 1.6 GB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | sf6 procapsid icosahedral reconstruction | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
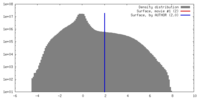
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Bacteriophage Sf6 procapsid
| Entire | Name: Bacteriophage Sf6 procapsid |
|---|---|
| Components |
|
-Supramolecule #1000: Bacteriophage Sf6 procapsid
| Supramolecule | Name: Bacteriophage Sf6 procapsid / type: sample / ID: 1000 / Oligomeric state: icosahedral / Number unique components: 1 |
|---|
-Supramolecule #1: Shigella phage Sf6
| Supramolecule | Name: Shigella phage Sf6 / type: virus / ID: 1 / Details: This is a precursor procapsid. / NCBI-ID: 10761 / Sci species name: Shigella phage Sf6 / Sci species strain: clear plaque mutant / Database: NCBI / Virus type: VIRUS-LIKE PARTICLE / Virus isolate: STRAIN / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism:  Shigella flexneri (bacteria) / Strain: PE577 / synonym: BACTERIA(EUBACTERIA) Shigella flexneri (bacteria) / Strain: PE577 / synonym: BACTERIA(EUBACTERIA) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 8 mg/mL |
|---|---|
| Buffer | pH: 7.6 / Details: 10mM Tris, 10mM MgCl2 |
| Grid | Details: 400 mesh Quantifoil R2/2, glow discharged |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 99 % / Chamber temperature: 90 K / Instrument: HOMEMADE PLUNGER / Method: Blot for 5 sec before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Temperature | Min: 89 K / Max: 91 K / Average: 90 K |
| Alignment procedure | Legacy - Astigmatism: objective lens astigmatism was corrected at high magnification |
| Date | Apr 19, 2011 |
| Image recording | Category: FILM / Film or detector model: KODAK SO-163 FILM / Digitization - Scanner: OTHER / Digitization - Sampling interval: 1.07 µm / Number real images: 139 / Average electron dose: 22 e/Å2 / Bits/pixel: 8 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated magnification: 58050 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.3 mm / Nominal defocus max: 4.61 µm / Nominal defocus min: 0.58 µm / Nominal magnification: 59000 |
| Sample stage | Specimen holder model: OTHER |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
- Image processing
Image processing
| Details | the reconstruction was done using AUTO3DEM |
|---|---|
| CTF correction | Details: Robem |
| Final reconstruction | Algorithm: OTHER / Resolution.type: BY AUTHOR / Resolution: 7.0 Å / Resolution method: FSC 0.5 CUT-OFF / Software - Name: Auto3dem Details: The particles were selected and preprocessed using RobEM. Image reconstruction was performed using Auto3DEM. Number images used: 6851 |
 Movie
Movie Controller
Controller
















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)