[English] 日本語
 Yorodumi
Yorodumi- EMDB-50128: Mammalian quaternary complex of a translating 80S ribosome, NAC, ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Mammalian quaternary complex of a translating 80S ribosome, NAC, MetAP1 and NatA/E - local refinement | ||||||||||||||||||
 Map data Map data | map of the quaternary RNC-NAC-MetAP1-NatAE complex refined with a mask on NatA/E-NAC-MetAP1 | ||||||||||||||||||
 Sample Sample |
| ||||||||||||||||||
 Keywords Keywords | translation / ribosome / NAC / N-terminal acetyltransferase / NatA / NatE / MetAP1 | ||||||||||||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | ||||||||||||||||||
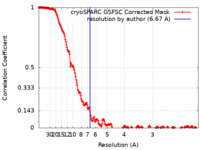
| Method | single particle reconstruction / cryo EM / Resolution: 6.67 Å | ||||||||||||||||||
 Authors Authors | Yudin D / Scaiola A / Ban N | ||||||||||||||||||
| Funding support |  United States, United States,  Germany, Germany,  Switzerland, European Union, 5 items Switzerland, European Union, 5 items
| ||||||||||||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: NAC guides a ribosomal multienzyme complex for nascent protein processing. Authors: Alfred M Lentzsch / Denis Yudin / Martin Gamerdinger / Sowmya Chandrasekar / Laurenz Rabl / Alain Scaiola / Elke Deuerling / Nenad Ban / Shu-Ou Shan /    Abstract: Approximately 40% of the mammalian proteome undergoes N-terminal methionine excision and acetylation, mediated sequentially by methionine aminopeptidase (MetAP) and N-acetyltransferase A (NatA), ...Approximately 40% of the mammalian proteome undergoes N-terminal methionine excision and acetylation, mediated sequentially by methionine aminopeptidase (MetAP) and N-acetyltransferase A (NatA), respectively. Both modifications are strictly cotranslational and essential in higher eukaryotic organisms. The interaction, activity and regulation of these enzymes on translating ribosomes are poorly understood. Here we perform biochemical, structural and in vivo studies to demonstrate that the nascent polypeptide-associated complex (NAC) orchestrates the action of these enzymes. NAC assembles a multienzyme complex with MetAP1 and NatA early during translation and pre-positions the active sites of both enzymes for timely sequential processing of the nascent protein. NAC further releases the inhibitory interactions from the NatA regulatory protein huntingtin yeast two-hybrid protein K (HYPK) to activate NatA on the ribosome, enforcing cotranslational N-terminal acetylation. Our results provide a mechanistic model for the cotranslational processing of proteins in eukaryotic cells. | ||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_50128.map.gz emd_50128.map.gz | 328.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-50128-v30.xml emd-50128-v30.xml emd-50128.xml emd-50128.xml | 44.7 KB 44.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_50128_fsc.xml emd_50128_fsc.xml | 18.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_50128.png emd_50128.png | 83.2 KB | ||
| Masks |  emd_50128_msk_1.map emd_50128_msk_1.map emd_50128_msk_2.map emd_50128_msk_2.map | 669.9 MB 669.9 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-50128.cif.gz emd-50128.cif.gz | 4.9 KB | ||
| Others |  emd_50128_additional_1.map.gz emd_50128_additional_1.map.gz emd_50128_additional_2.map.gz emd_50128_additional_2.map.gz emd_50128_half_map_1.map.gz emd_50128_half_map_1.map.gz emd_50128_half_map_2.map.gz emd_50128_half_map_2.map.gz | 321 MB 627.1 MB 621.1 MB 621.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-50128 http://ftp.pdbj.org/pub/emdb/structures/EMD-50128 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50128 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-50128 | HTTPS FTP |
-Validation report
| Summary document |  emd_50128_validation.pdf.gz emd_50128_validation.pdf.gz | 1.3 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_50128_full_validation.pdf.gz emd_50128_full_validation.pdf.gz | 1.3 MB | Display | |
| Data in XML |  emd_50128_validation.xml.gz emd_50128_validation.xml.gz | 27.7 KB | Display | |
| Data in CIF |  emd_50128_validation.cif.gz emd_50128_validation.cif.gz | 36.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50128 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50128 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50128 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-50128 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_50128.map.gz / Format: CCP4 / Size: 669.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_50128.map.gz / Format: CCP4 / Size: 669.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | map of the quaternary RNC-NAC-MetAP1-NatAE complex refined with a mask on NatA/E-NAC-MetAP1 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.065 Å | ||||||||||||||||||||||||||||||||||||
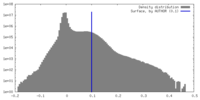
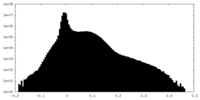
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_50128_msk_1.map emd_50128_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
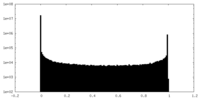
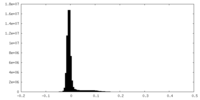
| Density Histograms |
-Mask #2
| File |  emd_50128_msk_2.map emd_50128_msk_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
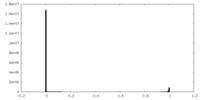
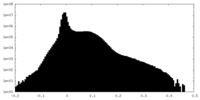
| Density Histograms |
-Additional map: resampled map used during the refinement of the...
| File | emd_50128_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | resampled map used during the refinement of the quaternary complex model - see the methods section of the manuscript for details | ||||||||||||
| Projections & Slices |
| ||||||||||||
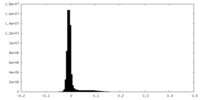
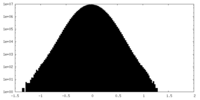
| Density Histograms |
-Additional map: main map lowpass filtered to 8 A resolution
| File | emd_50128_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | main map lowpass filtered to 8 A resolution | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: halfmap B
| File | emd_50128_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | halfmap B | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: halfmap A
| File | emd_50128_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | halfmap A | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Quaternary complex of a translating 80S ribosome, NAC, MetAP1 and...
| Entire | Name: Quaternary complex of a translating 80S ribosome, NAC, MetAP1 and NatA/E |
|---|---|
| Components |
|
-Supramolecule #1: Quaternary complex of a translating 80S ribosome, NAC, MetAP1 and...
| Supramolecule | Name: Quaternary complex of a translating 80S ribosome, NAC, MetAP1 and NatA/E type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#90 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 3.49 MDa |
-Supramolecule #2: Rabbit ribosome-nascent chain complex
| Supramolecule | Name: Rabbit ribosome-nascent chain complex / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#6, #13-#90 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: NAC heterodimer
| Supramolecule | Name: NAC heterodimer / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #8-#9 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #4: Human NatA/E complex
| Supramolecule | Name: Human NatA/E complex / type: complex / ID: 4 / Parent: 1 / Macromolecule list: #10-#12 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #5: Human NatA complex
| Supramolecule | Name: Human NatA complex / type: complex / ID: 5 / Parent: 4 / Macromolecule list: #11-#12 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #6: Human NatE
| Supramolecule | Name: Human NatE / type: complex / ID: 6 / Parent: 4 / Macromolecule list: #10 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #7: Human methionine aminopeptidase 1
| Supramolecule | Name: Human methionine aminopeptidase 1 / type: complex / ID: 7 / Parent: 1 / Macromolecule list: #7 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE-PROPANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: OTHER / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.6 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)