+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-4404 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | eIF2B:eIF2 complex, phosphorylated on eIF2 alpha serine 52. | |||||||||||||||
 Map data Map data | eIF2B:eIF2 complex with eIF2 alpha phosphorylated on serine 52 | |||||||||||||||
 Sample Sample |
| |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of cellular response to amino acid starvation / Cellular response to mitochondrial stress / Recycling of eIF2:GDP / ABC-family proteins mediated transport / methionyl-initiator methionine tRNA binding / eukaryotic translation initiation factor 2B complex / eukaryotic translation initiation factor 2 complex / multi-eIF complex / selenocysteine metabolic process / eukaryotic 43S preinitiation complex ...negative regulation of cellular response to amino acid starvation / Cellular response to mitochondrial stress / Recycling of eIF2:GDP / ABC-family proteins mediated transport / methionyl-initiator methionine tRNA binding / eukaryotic translation initiation factor 2B complex / eukaryotic translation initiation factor 2 complex / multi-eIF complex / selenocysteine metabolic process / eukaryotic 43S preinitiation complex / protein-synthesizing GTPase / cytoplasmic translational initiation / formation of cytoplasmic translation initiation complex / selenocysteine insertion sequence binding / formation of translation preinitiation complex / guanyl-nucleotide exchange factor complex / positive regulation of cellular response to amino acid starvation / eukaryotic 48S preinitiation complex / positive regulation of translational fidelity / regulation of translational initiation / Formation of the ternary complex, and subsequently, the 43S complex / Translation initiation complex formation / Ribosomal scanning and start codon recognition / L13a-mediated translational silencing of Ceruloplasmin expression / enzyme regulator activity / translation initiation factor binding / translational initiation / translation initiation factor activity / guanyl-nucleotide exchange factor activity / cytoplasmic stress granule / ribosome binding / ribosome / GTPase activity / mRNA binding / GTP binding / mitochondrion / RNA binding / metal ion binding / cytoplasm / cytosol Similarity search - Function | |||||||||||||||
| Biological species |   | |||||||||||||||
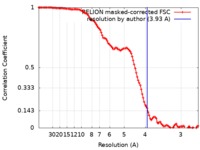
| Method | single particle reconstruction / cryo EM / Resolution: 3.93 Å | |||||||||||||||
 Authors Authors | Adomavicius T / Roseman AM / Pavitt GD | |||||||||||||||
| Funding support |  United Kingdom, 4 items United Kingdom, 4 items
| |||||||||||||||
 Citation Citation | Journal: Elife / Year: 2017 Title: Fail-safe control of translation initiation by dissociation of eIF2α phosphorylated ternary complexes. Authors: Martin D Jennings / Christopher J Kershaw / Tomas Adomavicius / Graham D Pavitt /  Abstract: Phosphorylation of eIF2α controls translation initiation by restricting the levels of active eIF2-GTP/Met-tRNAi ternary complexes (TC). This modulates the expression of all eukaryotic mRNAs and ...Phosphorylation of eIF2α controls translation initiation by restricting the levels of active eIF2-GTP/Met-tRNAi ternary complexes (TC). This modulates the expression of all eukaryotic mRNAs and contributes to the cellular integrated stress response. Key to controlling the activity of eIF2 are translation factors eIF2B and eIF5, thought to primarily function with eIF2-GDP and TC respectively. Using a steady-state kinetics approach with purified proteins we demonstrate that eIF2B binds to eIF2 with equal affinity irrespective of the presence or absence of competing guanine nucleotides. We show that eIF2B can compete with Met-tRNAi for eIF2-GTP and can destabilize TC. When TC is formed with unphosphorylated eIF2, eIF5 can out-compete eIF2B to stabilize TC/eIF5 complexes. However when TC/eIF5 is formed with phosphorylated eIF2, eIF2B outcompetes eIF5 and destabilizes TC. These data uncover competition between eIF2B and eIF5 for TC and identify that phosphorylated eIF2-GTP translation initiation intermediate complexes can be inhibited by eIF2B. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_4404.map.gz emd_4404.map.gz | 117.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-4404-v30.xml emd-4404-v30.xml emd-4404.xml emd-4404.xml | 36.9 KB 36.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_4404_fsc.xml emd_4404_fsc.xml | 11.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_4404.png emd_4404.png | 218.6 KB | ||
| Masks |  emd_4404_msk_1.map emd_4404_msk_1.map | 125 MB |  Mask map Mask map | |
| Others |  emd_4404_additional.map.gz emd_4404_additional.map.gz emd_4404_half_map_1.map.gz emd_4404_half_map_1.map.gz emd_4404_half_map_2.map.gz emd_4404_half_map_2.map.gz | 58.4 MB 97.1 MB 97.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-4404 http://ftp.pdbj.org/pub/emdb/structures/EMD-4404 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4404 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-4404 | HTTPS FTP |
-Validation report
| Summary document |  emd_4404_validation.pdf.gz emd_4404_validation.pdf.gz | 454.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_4404_full_validation.pdf.gz emd_4404_full_validation.pdf.gz | 454 KB | Display | |
| Data in XML |  emd_4404_validation.xml.gz emd_4404_validation.xml.gz | 16.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4404 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4404 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4404 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-4404 | HTTPS FTP |
-Related structure data
| Related structure data |  6i3mMC  4428C  6i7tC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_4404.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_4404.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | eIF2B:eIF2 complex with eIF2 alpha phosphorylated on serine 52 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.34 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
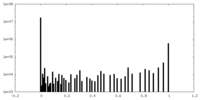
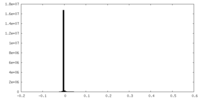
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_4404_msk_1.map emd_4404_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
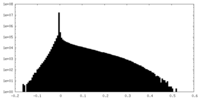
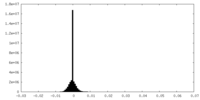
| Density Histograms |
-Additional map: LocScale version of main map
| File | emd_4404_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | LocScale version of main map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: relion half-map 1
| File | emd_4404_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | relion half-map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: relion half-map 2
| File | emd_4404_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | relion half-map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of translation initiation factors eIF2 and eIF2B
| Entire | Name: Complex of translation initiation factors eIF2 and eIF2B |
|---|---|
| Components |
|
-Supramolecule #1: Complex of translation initiation factors eIF2 and eIF2B
| Supramolecule | Name: Complex of translation initiation factors eIF2 and eIF2B type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
| Recombinant expression | Organism:  |
| Molecular weight | Experimental: 838 KDa |
-Macromolecule #1: Translation initiation factor eIF-2B subunit alpha
| Macromolecule | Name: Translation initiation factor eIF-2B subunit alpha / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 34.062027 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSEFNITETY LRFLEEDTEM TMPIAAIEAL VTLLRIKTPE TAAEMINTIK SSTEELIKSI PNSVSLRAGC DIFMRFVLRN LHLYGDWEN CKQHLIENGQ LFVSRAKKSR NKIAEIGVDF IADDDIILVH GYSRAVFSLL NHAANKFIRF RCVVTESRPS K QGNQLYTL ...String: MSEFNITETY LRFLEEDTEM TMPIAAIEAL VTLLRIKTPE TAAEMINTIK SSTEELIKSI PNSVSLRAGC DIFMRFVLRN LHLYGDWEN CKQHLIENGQ LFVSRAKKSR NKIAEIGVDF IADDDIILVH GYSRAVFSLL NHAANKFIRF RCVVTESRPS K QGNQLYTL LEQKGIPVTL IVDSAVGAVI DKVDKVFVGA EGVAESGGII NLVGTYSVGV LAHNARKPFY VVTESHKFVR MF PLSSDDL PMAGPPLDFT RRTDDLEDAL RGPTIDYTAQ EYITALITDL GVLTPSAVSE ELIKMWYD |
-Macromolecule #2: Translation initiation factor eIF-2B subunit delta
| Macromolecule | Name: Translation initiation factor eIF-2B subunit delta / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 70.945195 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSESEAKSRS ATPPSKAKQA TPTTTAAANG EKKLTNKELK ELKKQEKAAK RAAMKQANGI SIEQQQQQAQ MKKEKKQLQR EQQQKREQK QKNANKKKQN ERNVKKSTLF GHLETTEERR ATILALTSAV SSPKTSRITA AGLMVPVVAS ALSGSNVLTA S SLMPVGPN ...String: MSESEAKSRS ATPPSKAKQA TPTTTAAANG EKKLTNKELK ELKKQEKAAK RAAMKQANGI SIEQQQQQAQ MKKEKKQLQR EQQQKREQK QKNANKKKQN ERNVKKSTLF GHLETTEERR ATILALTSAV SSPKTSRITA AGLMVPVVAS ALSGSNVLTA S SLMPVGPN ASSTVSASAP ASTTTTLPAS SAALSAGTSS ASTNTPTAIQ QEIASSNASD VAKTLASISL EAGEFNVIPG IS SVIPTVL EQSFDNSSLI SSVKELLLNK DLIHPSILLL TSHLAHYKIV GSIPRCIAML EVFQIVIKDY QTPKGTTLSR NLT SYLSHQ IDLLKKARPL SVTMGNAIRW LKQEISLIDP STPDKAAKKD LCEKIGQFAK EKIELADQLI IDNASTQIEE STTI VTYGS SKVLTELLLH NAISLKKNIK VIVVDSRPLF EGRKMAETLR NAGVNVMYAL ITSLDTIFNM DVDYVFLGAH SILSN GFLY SRAGTAMLAM SAKRRNIPVL VCCESLKFSQ RVQLDSVTFN ELADPNDLVN IDYENPVERR GNKGALLNQF IKERKF EKK KLAMENKPKG NKIGGKKGSE GESKDASNEE DSNSKNILDG WQELPSLNIV NILYDLTPPE YIKKVITEFG ALPPSSV PV ILREYKGSA |
-Macromolecule #3: Translation initiation factor eIF-2B subunit beta
| Macromolecule | Name: Translation initiation factor eIF-2B subunit beta / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 42.621441 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSSQAFTSVH PNAATSDVNV TIDTFVAKLK RRQVQGSYAI ALETLQLLMR FISAARWNHV NDLIEQIRDL GNSLEKAHPT AFSCGNVIR RILAVLRDEV EEDTMSTTVT STSVAEPLIS SMFNLLQKPE QPHQNRKNSS GSSSMKTKTD YRQVAIQGIK D LIDEIKNI ...String: MSSQAFTSVH PNAATSDVNV TIDTFVAKLK RRQVQGSYAI ALETLQLLMR FISAARWNHV NDLIEQIRDL GNSLEKAHPT AFSCGNVIR RILAVLRDEV EEDTMSTTVT STSVAEPLIS SMFNLLQKPE QPHQNRKNSS GSSSMKTKTD YRQVAIQGIK D LIDEIKNI DEGIQQIAID LIHDHEILLT PTPDSKTVLK FLITARERSN RTFTVLVTEG FPNNTKNAHE FAKKLAQHNI ET LVVPDSA VFALMSRVGK VIIGTKAVFV NGGTISSNSG VSSVCECARE FRTPVFAVAG LYKLSPLYPF DVEKFVEFGG SQR ILPRMD PRKRLDTVNQ ITDYVPPENI DIYITNVGGF NPSFIYRIAW DNYKQIDVHL DKNKA |
-Macromolecule #4: Translation initiation factor eIF-2B subunit epsilon
| Macromolecule | Name: Translation initiation factor eIF-2B subunit epsilon / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 81.249062 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAGKKGQKKS GLGNHGKNSD MDVEDRLQAV VLTDSYETRF MPLTAVKPRC LLPLANVPLI EYTLEFLAKA GVHEVFLICS SHANQINDY IENSKWNLPW SPFKITTIMS PEARCTGDVM RDLDNRGIIT GDFILVSGDV LTNIDFSKML EFHKKMHLQD K DHISTMCL ...String: MAGKKGQKKS GLGNHGKNSD MDVEDRLQAV VLTDSYETRF MPLTAVKPRC LLPLANVPLI EYTLEFLAKA GVHEVFLICS SHANQINDY IENSKWNLPW SPFKITTIMS PEARCTGDVM RDLDNRGIIT GDFILVSGDV LTNIDFSKML EFHKKMHLQD K DHISTMCL SKASTYPKTR TIEPAAFVLD KSTSRCIYYQ DLPLPSSREK TSIQIDPELL DNVDEFVIRN DLIDCRIDIC TS HVPLIFQ ENFDYQSLRT DFVKGVISSD ILGKHIYAYL TDEYAVRVES WQTYDTISQD FLGRWCYPLV LDSNIQDDQT YSY ESRHIY KEKDVVLAQS CKIGKCTAIG SGTKIGEGTK IENSVIGRNC QIGENIRIKN SFIWDDCIIG NNSIIDHSLI ASNA TLGSN VRLNDGCIIG FNVKIDDNMD LDRNTKISAS PLKNAGSRMY DNESNEQFDQ DLDDQTLAVS IVGDKGVGYI YESEV SDDE DSSTEACKEI NTLSNQLDEL YLSDDSISSA TKKTKKRRTM SVNSIYTDRE EIDSEFEDED FEKEGIATVE RAMENN HDL DTALLELNTL RMSMNVTYHE VRIATITALL RRVYHFIATQ TLGPKDAVVK VFNQWGLLFK RQAFDEEEYI DLMNIIM EK IVEQSFDKPD LILFSALVSL YDNDIIEEDV IYKWWDNVST DPRYDEVKKL TVKWVEWLQN ADEESSSEEE |
-Macromolecule #5: Translation initiation factor eIF-2B subunit gamma
| Macromolecule | Name: Translation initiation factor eIF-2B subunit gamma / type: protein_or_peptide / ID: 5 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 65.76832 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSIQAFVFCG KGSNLAPFTQ PDFPFQTQNK DSTAATSGDK LNELVNSALD STVINEFMQH STRLPKALLP IGNRPMIEYV LDWCDQADF KEISVVAPVD EIELIESGLT SFLSLRKQQF ELIYKALSNS NHSHHLQDPK KINFIPSKAN STGESLQKEL L PRINGDFV ...String: MSIQAFVFCG KGSNLAPFTQ PDFPFQTQNK DSTAATSGDK LNELVNSALD STVINEFMQH STRLPKALLP IGNRPMIEYV LDWCDQADF KEISVVAPVD EIELIESGLT SFLSLRKQQF ELIYKALSNS NHSHHLQDPK KINFIPSKAN STGESLQKEL L PRINGDFV ILPCDFVTDI PPQVLVDQFR NRDDNNLAMT IYYKNSLDSS IDKKQQQKQK QQQFFTVYSE NEDSERQPIL LD VYSQRDV TKTKYLQIRS HLLWNYPNLT VSTKLLNSFI YFCSFELCQL LKLGPQSMSR QASFKDPFTG NQQQQNPPTT DDD EDRNHD DDDDYKPSAT SIQPTYFKKK NDLILDPINC NKSLSKVFRD LSRRSWQHSK PREPIGIFIL PNETLFIRAN NLNA YMDAN RFVLKIKSQT MFTKNIQIQS AAIGADAIVD PKCQISAHSN VKMSVLGTQA NIGSRCRVAG SLLFPGVHLG DEVIL ENCI IGPMAKIGSK CKLSNCYIEG HYVVEPKNNF KGETLANVYL DEDEEDELIY DDSVIAGESE IAEETDSDDR SDEDSD DSE YTDEYEYEDD GLFER |
-Macromolecule #6: Eukaryotic translation initiation factor 2 subunit alpha
| Macromolecule | Name: Eukaryotic translation initiation factor 2 subunit alpha type: protein_or_peptide / ID: 6 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 34.843633 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSTSHCRFYE NKYPEIDDIV MVNVQQIAEM GAYVKLLEYD NIEGMILLSE L(SEP)RRRIRSIQ KLIRVGKNDV AVVLRV DKE KGYIDLSKRR VSSEDIIKCE EKYQKSKTVH SILRYCAEKF QIPLEELYKT IAWPLSRKFG HAYEAFKLSI IDETVWE GI EPPSKDVLDE ...String: MSTSHCRFYE NKYPEIDDIV MVNVQQIAEM GAYVKLLEYD NIEGMILLSE L(SEP)RRRIRSIQ KLIRVGKNDV AVVLRV DKE KGYIDLSKRR VSSEDIIKCE EKYQKSKTVH SILRYCAEKF QIPLEELYKT IAWPLSRKFG HAYEAFKLSI IDETVWE GI EPPSKDVLDE LKNYISKRLT PQAVKIRADV EVSCFSYEGI DAIKDALKSA EDMSTEQMQV KVKLVAAPLY VLTTQALD K QKGIEQLESA IEKITEVITK YGGVCNITMP PKAVTATEDA ELQALLESKE LDNRSDSEDD EDESDDE |
-Macromolecule #7: Eukaryotic translation initiation factor 2 subunit beta
| Macromolecule | Name: Eukaryotic translation initiation factor 2 subunit beta type: protein_or_peptide / ID: 7 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 31.631309 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSSDLAAELG FDPALKKKKK TKKVIPDDFD AAVNGKENGS GDDLFAGLKK KKKKSKSVSA DAEAEKEPTD DIAEALGELS LKKKKKKTK DSSVDAFEKE LAKAGLDNVD AESKEGTPSA NSSIQQEVGL PYSELLSRFF NILRTNNPEL AGDRSGPKFR I PPPVCLRD ...String: MSSDLAAELG FDPALKKKKK TKKVIPDDFD AAVNGKENGS GDDLFAGLKK KKKKSKSVSA DAEAEKEPTD DIAEALGELS LKKKKKKTK DSSVDAFEKE LAKAGLDNVD AESKEGTPSA NSSIQQEVGL PYSELLSRFF NILRTNNPEL AGDRSGPKFR I PPPVCLRD GKKTIFSNIQ DIAEKLHRSP EHLIQYLFAE LGTSGSVDGQ KRLVIKGKFQ SKQMENVLRR YILEYVTCKT CK SINTELK REQSNRLFFM VCKSCGSTRS VSSIKTGFQA TVGKRRRM |
-Macromolecule #8: Eukaryotic translation initiation factor 2 subunit gamma
| Macromolecule | Name: Eukaryotic translation initiation factor 2 subunit gamma type: protein_or_peptide / ID: 8 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 57.942699 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSDLQDQEPS IIINGNLEPV GEPDIVEETE VVAQETQETQ DADKPKKKVA FTGLEEDGET EEEKRKREFE EGGGLPEQPL NPDFSKLNP LSAEIINRQA TINIGTIGHV AHGKSTVVRA ISGVQTVRFK DELERNITIK LGYANAKIYK CQEPTCPEPD C YRSFKSDK ...String: MSDLQDQEPS IIINGNLEPV GEPDIVEETE VVAQETQETQ DADKPKKKVA FTGLEEDGET EEEKRKREFE EGGGLPEQPL NPDFSKLNP LSAEIINRQA TINIGTIGHV AHGKSTVVRA ISGVQTVRFK DELERNITIK LGYANAKIYK CQEPTCPEPD C YRSFKSDK EISPKCQRPG CPGRYKLVRH VSFVDCPGHD ILMSTMLSGA AVMDAALLLI AGNESCPQPQ TSEHLAAIEI MK LKHVIIL QNKVDLMREE SALEHQKSIL KFIRGTIADG APIVPISAQL KYNIDAVNEF IVKTIPVPPR DFMISPRLIV IRS FDVNKP GAEIEDLKGG VAGGSILNGV FKLGDEIEIR PGIVTKDDKG KIQCKPIFSN IVSLFAEQND LKFAVPGGLI GVGT KVDPT LCRADRLVGQ VVGAKGHLPN IYTDIEINYF LLRRLLGVKT DGQKQAKVRK LEPNEVLMVN IGSTATGARV VAVKA DMAR LQLTSPACTE INEKIALSRR IEKHWRLIGW ATIKKGTTLE PIA |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.25 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
Details: Solutions for sample preparation were made fresh, filter sterilized, and degassed. | ||||||||||||
| Grid | Model: Homemade / Material: COPPER / Mesh: 400 / Support film - #0 - Film type ID: 1 / Support film - #0 - Material: CARBON / Support film - #0 - topology: LACEY / Support film - #1 - Film type ID: 2 / Support film - #1 - Material: CARBON / Support film - #1 - topology: CONTINUOUS / Support film - #1 - Film thickness: 3.0 nm / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 101.325 kPa Details: Ultra-thin carbon support film, 3nm - on lacey carbon grids from Agar Scientific were used for 35 degree tilted data collection. For zero tilt data collection, 200 mesh Au Quantifoil, R2/2 grids were used. | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 294 K / Instrument: FEI VITROBOT MARK III / Details: Blot for 2 seconds before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Quantum LS / Energy filter - Slit width: 20 eV |
| Details | Tilt at zero and 35 degrees. |
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 3838 pixel / Digitization - Dimensions - Height: 3710 pixel / Digitization - Sampling interval: 5.0 µm / Digitization - Frames/image: 2-48 / Number grids imaged: 2 / Number real images: 4533 / Average exposure time: 12.0 sec. / Average electron dose: 40.0 e/Å2 Details: 2 separate data collections for zero degree (2255 images) and tilted (2278 images) specimen. Both on Titan Krios |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Calibrated defocus max: 5.5 µm / Calibrated defocus min: 1.0 µm / Calibrated magnification: 37313 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 4.0 µm / Nominal defocus min: 1.5 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Details | Modeller was used to build homology model of S. cerevisiae eIF2B structure based on S. pombe crystal structure. Subunits of the homology model, along with eIF2 alpha domains 1 and 2, were then rigid body fitted into our map using UCSF Chimera. The model then was refined using phenix and manually adjusted in Coot. | ||||||
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Target criteria: correlation coeficient | ||||||
| Output model |  PDB-6i3m: |
-Atomic model buiding 2
| Initial model |
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Details | eIF2 alpha domain 3, eIF2 gamma, and eIF2 beta (3JAP) were rigid body fitted into our map using Chimera. | ||||||||
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Target criteria: Cross-correlation coefficient | ||||||||
| Output model |  PDB-6i3m: |
 Movie
Movie Controller
Controller





























 Z
Z Y
Y X
X