+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | In-situ complex I with Q10 (State-gamma) | |||||||||
 Map data Map data | In-situ complex I with Q10intermediate-II | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | in-situ cryo-EM structure / mammalian / mitochondria / respiratory supercomplex / proton pumping / membrane protein / electron transport | |||||||||
| Function / homology |  Function and homology information Function and homology informationRHOG GTPase cycle / Complex I biogenesis / Respiratory electron transport / Neutrophil degranulation / Mitochondrial protein degradation / oxidoreductase activity, acting on NAD(P)H / cardiac muscle tissue development / cellular respiration / ubiquinone binding / NADH:ubiquinone reductase (H+-translocating) ...RHOG GTPase cycle / Complex I biogenesis / Respiratory electron transport / Neutrophil degranulation / Mitochondrial protein degradation / oxidoreductase activity, acting on NAD(P)H / cardiac muscle tissue development / cellular respiration / ubiquinone binding / NADH:ubiquinone reductase (H+-translocating) / NADH dehydrogenase activity / respiratory chain complex I / : / mitochondrial electron transport, NADH to ubiquinone / mitochondrial respiratory chain complex I assembly / electron transport coupled proton transport / acyl binding / acyl carrier activity / NADH dehydrogenase (ubiquinone) activity / negative regulation of intrinsic apoptotic signaling pathway / ATP synthesis coupled electron transport / quinone binding / : / response to cAMP / aerobic respiration / respiratory electron transport chain / reactive oxygen species metabolic process / electron transport chain / brain development / regulation of protein phosphorylation / negative regulation of cell growth / mitochondrial intermembrane space / 2 iron, 2 sulfur cluster binding / positive regulation of fibroblast proliferation / NAD binding / FMN binding / nervous system development / 4 iron, 4 sulfur cluster binding / response to oxidative stress / mitochondrial inner membrane / nuclear body / mitochondrial matrix / mitochondrion / nucleoplasm / metal ion binding / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.5 Å | |||||||||
 Authors Authors | Zheng W / Zhu J / Zhang K | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2024 Journal: Nature / Year: 2024Title: High-resolution in situ structures of mammalian respiratory supercomplexes. Authors: Wan Zheng / Pengxin Chai / Jiapeng Zhu / Kai Zhang /   Abstract: Mitochondria play a pivotal part in ATP energy production through oxidative phosphorylation, which occurs within the inner membrane through a series of respiratory complexes. Despite extensive in ...Mitochondria play a pivotal part in ATP energy production through oxidative phosphorylation, which occurs within the inner membrane through a series of respiratory complexes. Despite extensive in vitro structural studies, determining the atomic details of their molecular mechanisms in physiological states remains a major challenge, primarily because of loss of the native environment during purification. Here we directly image porcine mitochondria using an in situ cryo-electron microscopy approach. This enables us to determine the structures of various high-order assemblies of respiratory supercomplexes in their native states. We identify four main supercomplex organizations: IIIIIV, IIIIIV, IIIIIV and IIIIIV, which potentially expand into higher-order arrays on the inner membranes. These diverse supercomplexes are largely formed by 'protein-lipids-protein' interactions, which in turn have a substantial impact on the local geometry of the surrounding membranes. Our in situ structures also capture numerous reactive intermediates within these respiratory supercomplexes, shedding light on the dynamic processes of the ubiquinone/ubiquinol exchange mechanism in complex I and the Q-cycle in complex III. Structural comparison of supercomplexes from mitochondria treated under different conditions indicates a possible correlation between conformational states of complexes I and III, probably in response to environmental changes. By preserving the native membrane environment, our approach enables structural studies of mitochondrial respiratory supercomplexes in reaction at high resolution across multiple scales, from atomic-level details to the broader subcellular context. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_42168.map.gz emd_42168.map.gz | 63 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-42168-v30.xml emd-42168-v30.xml emd-42168.xml emd-42168.xml | 65.1 KB 65.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_42168.png emd_42168.png | 62.8 KB | ||
| Filedesc metadata |  emd-42168.cif.gz emd-42168.cif.gz | 14.2 KB | ||
| Others |  emd_42168_half_map_1.map.gz emd_42168_half_map_1.map.gz emd_42168_half_map_2.map.gz emd_42168_half_map_2.map.gz | 116.1 MB 116 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-42168 http://ftp.pdbj.org/pub/emdb/structures/EMD-42168 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42168 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-42168 | HTTPS FTP |
-Validation report
| Summary document |  emd_42168_validation.pdf.gz emd_42168_validation.pdf.gz | 1.2 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_42168_full_validation.pdf.gz emd_42168_full_validation.pdf.gz | 1.2 MB | Display | |
| Data in XML |  emd_42168_validation.xml.gz emd_42168_validation.xml.gz | 13.7 KB | Display | |
| Data in CIF |  emd_42168_validation.cif.gz emd_42168_validation.cif.gz | 16.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42168 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42168 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42168 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-42168 | HTTPS FTP |
-Related structure data
| Related structure data |  8uerMC  8ud1C  8ueoC  8uepC  8ueqC  8uesC  8uetC  8ueuC  8uevC  8uewC  8uexC  8ueyC  8uezC  8ugdC  8ugeC  8ugfC  8uggC  8ughC  8ugiC  8ugjC  8ugkC  8uglC  8ugnC  8ugpC  8ugrC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_42168.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_42168.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | In-situ complex I with Q10intermediate-II | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.39 Å | ||||||||||||||||||||||||||||||||||||
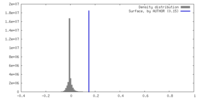
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: In-situ complex I with Q10intermediate-II
| File | emd_42168_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | In-situ complex I with Q10intermediate-II | ||||||||||||
| Projections & Slices |
| ||||||||||||
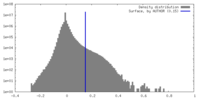
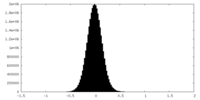
| Density Histograms |
-Half map: In-situ complex I with Q10intermediate-II
| File | emd_42168_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | In-situ complex I with Q10intermediate-II | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : In-situ cryo-EM structure of respiratory supercomplex by directly...
+Supramolecule #1: In-situ cryo-EM structure of respiratory supercomplex by directly...
+Macromolecule #1: NADH-ubiquinone oxidoreductase chain 3
+Macromolecule #2: NADH dehydrogenase [ubiquinone] iron-sulfur protein 7, mitochondrial
+Macromolecule #3: NADH dehydrogenase [ubiquinone] iron-sulfur protein 3, mitochondrial
+Macromolecule #4: NADH dehydrogenase [ubiquinone] iron-sulfur protein 2, mitochondrial
+Macromolecule #5: NADH dehydrogenase [ubiquinone] flavoprotein 2, mitochondrial
+Macromolecule #6: NADH dehydrogenase [ubiquinone] flavoprotein 1, mitochondrial
+Macromolecule #7: NADH-ubiquinone oxidoreductase 75 kDa subunit, mitochondrial
+Macromolecule #8: NADH-ubiquinone oxidoreductase chain 1
+Macromolecule #9: NADH dehydrogenase [ubiquinone] iron-sulfur protein 8, mitochondrial
+Macromolecule #10: NADH-ubiquinone oxidoreductase chain 6
+Macromolecule #11: NADH-ubiquinone oxidoreductase chain 4L
+Macromolecule #12: NADH-ubiquinone oxidoreductase chain 5
+Macromolecule #13: NADH-ubiquinone oxidoreductase chain 4
+Macromolecule #14: NADH-ubiquinone oxidoreductase chain 2
+Macromolecule #15: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 10, mi...
+Macromolecule #16: NADH:ubiquinone oxidoreductase subunit A9
+Macromolecule #17: NADH dehydrogenase [ubiquinone] iron-sulfur protein 4, mitochondrial
+Macromolecule #18: NADH dehydrogenase [ubiquinone] iron-sulfur protein 6, mitochondrial
+Macromolecule #19: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 2
+Macromolecule #20: NADH:ubiquinone oxidoreductase subunit AB1
+Macromolecule #21: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 5 isof...
+Macromolecule #22: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 6
+Macromolecule #23: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 8
+Macromolecule #24: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 11
+Macromolecule #25: NADH:ubiquinone oxidoreductase subunit A13
+Macromolecule #26: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 1
+Macromolecule #27: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 3
+Macromolecule #28: NADH dehydrogenase [ubiquinone] 1 subunit C1, mitochondrial
+Macromolecule #29: NADH dehydrogenase [ubiquinone] 1 subunit C2
+Macromolecule #30: NADH dehydrogenase [ubiquinone] iron-sulfur protein 5
+Macromolecule #31: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 1 [Sus ...
+Macromolecule #32: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 11, mit...
+Macromolecule #33: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 5, mito...
+Macromolecule #34: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 6
+Macromolecule #35: NADH:ubiquinone oxidoreductase subunit B2
+Macromolecule #36: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 3
+Macromolecule #37: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 8, mito...
+Macromolecule #38: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 4
+Macromolecule #39: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 9
+Macromolecule #40: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 7
+Macromolecule #41: NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 10
+Macromolecule #42: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 12
+Macromolecule #43: NADH dehydrogenase [ubiquinone] 1 alpha subcomplex subunit 7
+Macromolecule #44: NADH dehydrogenase [ubiquinone] flavoprotein 3, mitochondrial
+Macromolecule #45: IRON/SULFUR CLUSTER
+Macromolecule #46: 1,2-DIACYL-SN-GLYCERO-3-PHOSPHOCHOLINE
+Macromolecule #47: FE2/S2 (INORGANIC) CLUSTER
+Macromolecule #48: FLAVIN MONONUCLEOTIDE
+Macromolecule #49: POTASSIUM ION
+Macromolecule #50: UBIQUINONE-10
+Macromolecule #51: CARDIOLIPIN
+Macromolecule #52: 1,2-Distearoyl-sn-glycerophosphoethanolamine
+Macromolecule #53: GUANOSINE-5'-TRIPHOSPHATE
+Macromolecule #54: MAGNESIUM ION
+Macromolecule #55: NADPH DIHYDRO-NICOTINAMIDE-ADENINE-DINUCLEOTIDE PHOSPHATE
+Macromolecule #56: ZINC ION
+Macromolecule #57: ~{S}-[2-[3-[[(2~{R})-3,3-dimethyl-2-oxidanyl-4-phosphonooxy-butan...
+Macromolecule #58: MYRISTIC ACID
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.0 µm / Nominal defocus min: 1.3 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.5 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 45000 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller









































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)