[English] 日本語
 Yorodumi
Yorodumi- EMDB-41065: MERS-CoV Nsp1 protein bound to human 40S ribosome: 40S Body Focus... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | MERS-CoV Nsp1 protein bound to human 40S ribosome: 40S Body Focus Refined Map | |||||||||
 Map data Map data | 40S Body Focus Refined Map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | MERS-CoV Nsp1 / translation inhibition / 40S ribosome / betacoronaviruses / RIBOSOME | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.6 Å | |||||||||
 Authors Authors | Devarkar SC / Xiong Y | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2023 Journal: Cell Rep / Year: 2023Title: Structural basis for translation inhibition by MERS-CoV Nsp1 reveals a conserved mechanism for betacoronaviruses. Authors: Swapnil C Devarkar / Michael Vetick / Shravani Balaji / Ivan B Lomakin / Luojia Yang / Danni Jin / Wendy V Gilbert / Sidi Chen / Yong Xiong /  Abstract: All betacoronaviruses (β-CoVs) encode non-structural protein 1 (Nsp1), an essential pathogenicity factor that potently restricts host gene expression. Among the β-CoV family, MERS-CoV is the most ...All betacoronaviruses (β-CoVs) encode non-structural protein 1 (Nsp1), an essential pathogenicity factor that potently restricts host gene expression. Among the β-CoV family, MERS-CoV is the most distantly related member to SARS-CoV-2, and the mechanism for host translation inhibition by MERS-CoV Nsp1 remains controversial. Herein, we show that MERS-CoV Nsp1 directly interacts with the 40S ribosomal subunit. Using cryogenic electron microscopy (cryo-EM), we report a 2.6-Å structure of the MERS-CoV Nsp1 bound to the human 40S ribosomal subunit. The extensive interactions between C-terminal domain of MERS-CoV Nsp1 and the mRNA entry channel of the 40S ribosomal subunit are critical for its translation inhibition function. This mechanism of MERS-CoV Nsp1 is strikingly similar to SARS-CoV and SARS-CoV-2 Nsp1, despite modest sequence conservation. Our results reveal that the mechanism of host translation inhibition is conserved across β-CoVs and highlight a potential therapeutic target for the development of antivirals that broadly restrict β-CoVs. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41065.map.gz emd_41065.map.gz | 106 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41065-v30.xml emd-41065-v30.xml emd-41065.xml emd-41065.xml | 17.8 KB 17.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_41065_fsc.xml emd_41065_fsc.xml | 12.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_41065.png emd_41065.png | 134.9 KB | ||
| Filedesc metadata |  emd-41065.cif.gz emd-41065.cif.gz | 4 KB | ||
| Others |  emd_41065_half_map_1.map.gz emd_41065_half_map_1.map.gz emd_41065_half_map_2.map.gz emd_41065_half_map_2.map.gz | 194.5 MB 194.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41065 http://ftp.pdbj.org/pub/emdb/structures/EMD-41065 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41065 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41065 | HTTPS FTP |
-Validation report
| Summary document |  emd_41065_validation.pdf.gz emd_41065_validation.pdf.gz | 1021.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_41065_full_validation.pdf.gz emd_41065_full_validation.pdf.gz | 1021 KB | Display | |
| Data in XML |  emd_41065_validation.xml.gz emd_41065_validation.xml.gz | 21.5 KB | Display | |
| Data in CIF |  emd_41065_validation.cif.gz emd_41065_validation.cif.gz | 28 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41065 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41065 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41065 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-41065 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_41065.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41065.map.gz / Format: CCP4 / Size: 209.3 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | 40S Body Focus Refined Map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Halfmap A
| File | emd_41065_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Halfmap A | ||||||||||||
| Projections & Slices |
| ||||||||||||
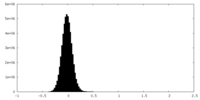
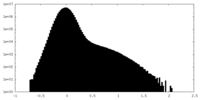
| Density Histograms |
-Half map: Halfmap B
| File | emd_41065_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Halfmap B | ||||||||||||
| Projections & Slices |
| ||||||||||||
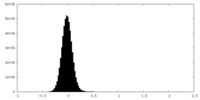
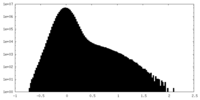
| Density Histograms |
- Sample components
Sample components
-Entire : MERS-CoV Nsp1 bound to human 40S ribosomal subunit
| Entire | Name: MERS-CoV Nsp1 bound to human 40S ribosomal subunit |
|---|---|
| Components |
|
-Supramolecule #1: MERS-CoV Nsp1 bound to human 40S ribosomal subunit
| Supramolecule | Name: MERS-CoV Nsp1 bound to human 40S ribosomal subunit / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#36 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 81000 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)