[English] 日本語
 Yorodumi
Yorodumi- EMDB-3769: Cryo-EM structure of a pre-catalytic human spliceosome primed for... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3769 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of a pre-catalytic human spliceosome primed for activation (B complex). Unmasked refinement, NON-sharpened map | |||||||||
 Map data Map data | NON-sharpened, unmasked refinement. Map was aligned to high resolution map (EMDB 3766) and RESAMPLED from 256 px boxsize to 432 px boxsize in Chimera to fit high-resolution map. | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 9.9 Å | |||||||||
 Authors Authors | Bertram K | |||||||||
 Citation Citation |  Journal: Cell / Year: 2017 Journal: Cell / Year: 2017Title: Cryo-EM Structure of a Pre-catalytic Human Spliceosome Primed for Activation. Authors: Karl Bertram / Dmitry E Agafonov / Olexandr Dybkov / David Haselbach / Majety N Leelaram / Cindy L Will / Henning Urlaub / Berthold Kastner / Reinhard Lührmann / Holger Stark /  Abstract: Little is known about the spliceosome's structure before its extensive remodeling into a catalytically active complex. Here, we report a 3D cryo-EM structure of a pre-catalytic human spliceosomal B ...Little is known about the spliceosome's structure before its extensive remodeling into a catalytically active complex. Here, we report a 3D cryo-EM structure of a pre-catalytic human spliceosomal B complex. The U2 snRNP-containing head domain is connected to the B complex main body via three main bridges. U4/U6.U5 tri-snRNP proteins, which are located in the main body, undergo significant rearrangements during tri-snRNP integration into the B complex. These include formation of a partially closed Prp8 conformation that creates, together with Dim1, a 5' splice site (ss) binding pocket, displacement of Sad1, and rearrangement of Brr2 such that it contacts its U4/U6 substrate and is poised for the subsequent spliceosome activation step. The molecular organization of several B-specific proteins suggests that they are involved in negatively regulating Brr2, positioning the U6/5'ss helix, and stabilizing the B complex structure. Our results indicate significant differences between the early activation phase of human and yeast spliceosomes. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3769.map.gz emd_3769.map.gz | 278.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3769-v30.xml emd-3769-v30.xml emd-3769.xml emd-3769.xml | 21.4 KB 21.4 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_3769.png emd_3769.png | 53.4 KB | ||
| Others |  emd_3769_additional.map.gz emd_3769_additional.map.gz emd_3769_half_map_1.map.gz emd_3769_half_map_1.map.gz emd_3769_half_map_2.map.gz emd_3769_half_map_2.map.gz | 49.2 MB 49.6 MB 49.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3769 http://ftp.pdbj.org/pub/emdb/structures/EMD-3769 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3769 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3769 | HTTPS FTP |
-Validation report
| Summary document |  emd_3769_validation.pdf.gz emd_3769_validation.pdf.gz | 331 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_3769_full_validation.pdf.gz emd_3769_full_validation.pdf.gz | 330.2 KB | Display | |
| Data in XML |  emd_3769_validation.xml.gz emd_3769_validation.xml.gz | 12.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3769 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3769 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3769 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3769 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_3769.map.gz / Format: CCP4 / Size: 307.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3769.map.gz / Format: CCP4 / Size: 307.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | NON-sharpened, unmasked refinement. Map was aligned to high resolution map (EMDB 3766) and RESAMPLED from 256 px boxsize to 432 px boxsize in Chimera to fit high-resolution map. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.16 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
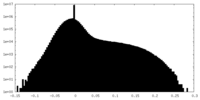
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: NON-sharpened, unmasked refinement. Map with original boxsize used...
| File | emd_3769_additional.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | NON-sharpened, unmasked refinement. Map with original boxsize used in refinement | ||||||||||||
| Projections & Slices |
| ||||||||||||
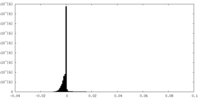
| Density Histograms |
-Half map: half-map 1
| File | emd_3769_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half-map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
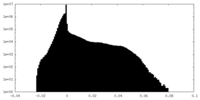
| Density Histograms |
-Half map: half-map 2
| File | emd_3769_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half-map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
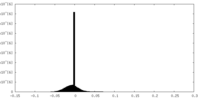
| Density Histograms |
- Sample components
Sample components
-Entire : Cryo-EM structure of a pre-catalytic human spliceosome primed for...
| Entire | Name: Cryo-EM structure of a pre-catalytic human spliceosome primed for activation (B complex). Unmasked refinement, NON-sharpened map |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of a pre-catalytic human spliceosome primed for...
| Supramolecule | Name: Cryo-EM structure of a pre-catalytic human spliceosome primed for activation (B complex). Unmasked refinement, NON-sharpened map type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#43 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.9 |
|---|---|
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 80 % / Chamber temperature: 277 K |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Digitization - Sampling interval: 14.0 µm / Digitization - Frames/image: 2-17 / Average exposure time: 0.05 sec. / Average electron dose: 1.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Cs: 0.01 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL |
|---|
 Movie
Movie Controller
Controller














 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)