+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-31897 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human p97 single hexamer conformer I with ATPgammaS bound | ||||||||||||
 Map data Map data | p97 SH I | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | AAA+ ATPase / unfoldase / CELL CYCLE / HYDROLASE | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of Lys63-specific deubiquitinase activity / flavin adenine dinucleotide catabolic process / positive regulation of oxidative phosphorylation / VCP-NSFL1C complex / endosome to lysosome transport via multivesicular body sorting pathway / protein-DNA covalent cross-linking repair / endoplasmic reticulum stress-induced pre-emptive quality control / cellular response to arsenite ion / Derlin-1 retrotranslocation complex / BAT3 complex binding ...positive regulation of Lys63-specific deubiquitinase activity / flavin adenine dinucleotide catabolic process / positive regulation of oxidative phosphorylation / VCP-NSFL1C complex / endosome to lysosome transport via multivesicular body sorting pathway / protein-DNA covalent cross-linking repair / endoplasmic reticulum stress-induced pre-emptive quality control / cellular response to arsenite ion / Derlin-1 retrotranslocation complex / BAT3 complex binding / positive regulation of protein K63-linked deubiquitination / deubiquitinase activator activity / aggresome assembly / regulation of protein localization to chromatin / vesicle-fusing ATPase / mitotic spindle disassembly / VCP-NPL4-UFD1 AAA ATPase complex / NADH metabolic process / cellular response to misfolded protein / stress granule disassembly / K48-linked polyubiquitin modification-dependent protein binding / positive regulation of mitochondrial membrane potential / negative regulation of protein localization to chromatin / ubiquitin-modified protein reader activity / retrograde protein transport, ER to cytosol / regulation of aerobic respiration / positive regulation of ATP biosynthetic process / regulation of synapse organization / ATPase complex / ubiquitin-specific protease binding / ubiquitin-like protein ligase binding / MHC class I protein binding / RHOH GTPase cycle / autophagosome maturation / HSF1 activation / polyubiquitin modification-dependent protein binding / proteasomal protein catabolic process / Protein methylation / endoplasmic reticulum to Golgi vesicle-mediated transport / translesion synthesis / interstrand cross-link repair / negative regulation of smoothened signaling pathway / ERAD pathway / ATP metabolic process / endoplasmic reticulum unfolded protein response / Attachment and Entry / lipid droplet / viral genome replication / proteasome complex / Josephin domain DUBs / N-glycan trimming in the ER and Calnexin/Calreticulin cycle / Hh mutants are degraded by ERAD / macroautophagy / Defective CFTR causes cystic fibrosis / Hedgehog ligand biogenesis / Translesion Synthesis by POLH / positive regulation of protein-containing complex assembly / establishment of protein localization / ABC-family proteins mediated transport / ADP binding / autophagy / cytoplasmic stress granule / Aggrephagy / positive regulation of non-canonical NF-kappaB signal transduction / positive regulation of protein catabolic process / activation of cysteine-type endopeptidase activity involved in apoptotic process / KEAP1-NFE2L2 pathway / azurophil granule lumen / double-strand break repair / Ovarian tumor domain proteases / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / positive regulation of canonical Wnt signaling pathway / E3 ubiquitin ligases ubiquitinate target proteins / Neddylation / site of double-strand break / cellular response to heat / ubiquitin-dependent protein catabolic process / regulation of apoptotic process / protein phosphatase binding / proteasome-mediated ubiquitin-dependent protein catabolic process / secretory granule lumen / ficolin-1-rich granule lumen / Attachment and Entry / protein ubiquitination / protein domain specific binding / intracellular membrane-bounded organelle / DNA repair / glutamatergic synapse / lipid binding / ubiquitin protein ligase binding / DNA damage response / Neutrophil degranulation / endoplasmic reticulum membrane / perinuclear region of cytoplasm / endoplasmic reticulum / ATP hydrolysis activity / protein-containing complex / RNA binding / extracellular exosome / extracellular region Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
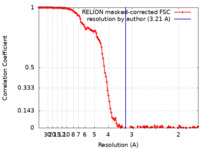
| Method | single particle reconstruction / cryo EM / Resolution: 3.21 Å | ||||||||||||
 Authors Authors | Gao H / Li F / Shi Z / Li Y / Yu H | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Cell Discov / Year: 2022 Journal: Cell Discov / Year: 2022Title: Cryo-EM structures of human p97 double hexamer capture potentiated ATPase-competent state. Authors: Haishan Gao / Faxiang Li / Zhejian Ji / Zhubing Shi / Yang Li / Hongtao Yu /   Abstract: The conserved ATPase p97 (Cdc48 in yeast) and adaptors mediate diverse cellular processes through unfolding polyubiquitinated proteins and extracting them from macromolecular assemblies and membranes ...The conserved ATPase p97 (Cdc48 in yeast) and adaptors mediate diverse cellular processes through unfolding polyubiquitinated proteins and extracting them from macromolecular assemblies and membranes for disaggregation and degradation. The tandem ATPase domains (D1 and D2) of the p97/Cdc48 hexamer form stacked rings. p97/Cdc48 can unfold substrates by threading them through the central pore. The pore loops critical for substrate unfolding are, however, not well-ordered in substrate-free p97/Cdc48 conformations. How p97/Cdc48 organizes its pore loops for substrate engagement is unclear. Here we show that p97/Cdc48 can form double hexamers (DH) connected through the D2 ring. Cryo-EM structures of p97 DH reveal an ATPase-competent conformation with ordered pore loops. The C-terminal extension (CTE) links neighboring D2s in each hexamer and expands the central pore of the D2 ring. Mutations of Cdc48 CTE abolish substrate unfolding. We propose that the p97/Cdc48 DH captures a potentiated state poised for substrate engagement. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_31897.map.gz emd_31897.map.gz | 95.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-31897-v30.xml emd-31897-v30.xml emd-31897.xml emd-31897.xml | 13.7 KB 13.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_31897_fsc.xml emd_31897_fsc.xml | 12.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_31897.png emd_31897.png | 106.6 KB | ||
| Filedesc metadata |  emd-31897.cif.gz emd-31897.cif.gz | 6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-31897 http://ftp.pdbj.org/pub/emdb/structures/EMD-31897 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31897 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31897 | HTTPS FTP |
-Validation report
| Summary document |  emd_31897_validation.pdf.gz emd_31897_validation.pdf.gz | 599.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_31897_full_validation.pdf.gz emd_31897_full_validation.pdf.gz | 598.7 KB | Display | |
| Data in XML |  emd_31897_validation.xml.gz emd_31897_validation.xml.gz | 12.5 KB | Display | |
| Data in CIF |  emd_31897_validation.cif.gz emd_31897_validation.cif.gz | 17 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31897 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31897 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31897 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31897 | HTTPS FTP |
-Related structure data
| Related structure data |  7vcvMC  7vcsC  7vctC  7vcuC  7vcxC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_31897.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_31897.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | p97 SH I | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : human p97 double hexamer conformer II with ATPgammaS bound
| Entire | Name: human p97 double hexamer conformer II with ATPgammaS bound |
|---|---|
| Components |
|
-Supramolecule #1: human p97 double hexamer conformer II with ATPgammaS bound
| Supramolecule | Name: human p97 double hexamer conformer II with ATPgammaS bound type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 1.2 MDa |
-Macromolecule #1: Transitional endoplasmic reticulum ATPase
| Macromolecule | Name: Transitional endoplasmic reticulum ATPase / type: protein_or_peptide / ID: 1 / Number of copies: 6 / Enantiomer: LEVO / EC number: vesicle-fusing ATPase |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 90.265711 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MASGADSKGD DLSTAILKQK NRPNRLIVDE AINEDNSVVS LSQPKMDELQ LFRGDTVLLK GKKRREAVCI VLSDDTCSDE KIRMNRVVR NNLRVRLGDV ISIQPCPDVK YGKRIHVLPI DDTVEGITGN LFEVYLKPYF LEAYRPIRKG DIFLVRGGMR A VEFKVVET ...String: MASGADSKGD DLSTAILKQK NRPNRLIVDE AINEDNSVVS LSQPKMDELQ LFRGDTVLLK GKKRREAVCI VLSDDTCSDE KIRMNRVVR NNLRVRLGDV ISIQPCPDVK YGKRIHVLPI DDTVEGITGN LFEVYLKPYF LEAYRPIRKG DIFLVRGGMR A VEFKVVET DPSPYCIVAP DTVIHCEGEP IKREDEEESL NEVGYDDIGG CRKQLAQIKE MVELPLRHPA LFKAIGVKPP RG ILLYGPP GTGKTLIARA VANETGAFFF LINGPEIMSK LAGESESNLR KAFEEAEKNA PAIIFIDELD AIAPKREKTH GEV ERRIVS QLLTLMDGLK QRAHVIVMAA TNRPNSIDPA LRRFGRFDRE VDIGIPDATG RLEILQIHTK NMKLADDVDL EQVA NETHG HVGADLAALC SEAALQAIRK KMDLIDLEDE TIDAEVMNSL AVTMDDFRWA LSQSNPSALR ETVVEVPQVT WEDIG GLED VKRELQELVQ YPVEHPDKFL KFGMTPSKGV LFYGPPGCGK TLLAKAIANE CQANFISIKG PELLTMWFGE SEANVR EIF DKARQAAPCV LFFDELDSIA KARGGNIGDG GGAADRVINQ ILTEMDGMST KKNVFIIGAT NRPDIIDPAI LRPGRLD QL IYIPLPDEKS RVAILKANLR KSPVAKDVDL EFLAKMTNGF SGADLTEICQ RACKLAIRES IESEIRRERE RQTNPSAM E VEEDDPVPEI RRDHFEEAMR FARRSVSDND IRKYEMFAQT LQQSRGFGSF RFPSGNQGGA GPSQGSGGGT GGSVYTEDN DDDLYGHHHH HH UniProtKB: Transitional endoplasmic reticulum ATPase |
-Macromolecule #2: PHOSPHOTHIOPHOSPHORIC ACID-ADENYLATE ESTER
| Macromolecule | Name: PHOSPHOTHIOPHOSPHORIC ACID-ADENYLATE ESTER / type: ligand / ID: 2 / Number of copies: 12 / Formula: AGS |
|---|---|
| Molecular weight | Theoretical: 523.247 Da |
| Chemical component information |  ChemComp-AGS: |
-Macromolecule #3: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 3 / Number of copies: 12 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.0 mg/mL |
|---|---|
| Buffer | pH: 7.5 Details: 25 mM HEPES-NaOH pH 7.5, 100 mM NaCl, 5 mM MgCl2, 0.5 mM TCEP, 0.01% NP40 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV Details: 3ul sample was applied and the grids were blotted for 3.0 s under 100% humidity at 277K before being plunged into liquid ethane using a Mark IV Vitrobot (FEI).. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average exposure time: 0.3 sec. / Average electron dose: 1.3 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller