[English] 日本語
 Yorodumi
Yorodumi- EMDB-29927: Domote-bound GluK2 kainate receptors in partially-open conformation 2 -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Domote-bound GluK2 kainate receptors in partially-open conformation 2 | |||||||||
 Map data Map data | Domote-bound GluK2 kainate receptors in partially-open conformation 2 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ion channel / glutamate kainate receptor 2 / homotetramer / MEMBRANE PROTEIN / partial agonist | |||||||||
| Function / homology |  Function and homology information Function and homology informationmossy fiber rosette / detection of cold stimulus involved in thermoception / Activation of Na-permeable kainate receptors / kainate selective glutamate receptor complex / Activation of Ca-permeable Kainate Receptor / negative regulation of synaptic transmission, glutamatergic / regulation of short-term neuronal synaptic plasticity / inhibitory postsynaptic potential / glutamate receptor activity / ubiquitin conjugating enzyme binding ...mossy fiber rosette / detection of cold stimulus involved in thermoception / Activation of Na-permeable kainate receptors / kainate selective glutamate receptor complex / Activation of Ca-permeable Kainate Receptor / negative regulation of synaptic transmission, glutamatergic / regulation of short-term neuronal synaptic plasticity / inhibitory postsynaptic potential / glutamate receptor activity / ubiquitin conjugating enzyme binding / receptor clustering / modulation of excitatory postsynaptic potential / regulation of JNK cascade / kainate selective glutamate receptor activity / ionotropic glutamate receptor complex / extracellularly glutamate-gated ion channel activity / neuronal action potential / behavioral fear response / positive regulation of synaptic transmission / glutamate-gated receptor activity / glutamate-gated calcium ion channel activity / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / presynaptic modulation of chemical synaptic transmission / dendrite cytoplasm / hippocampal mossy fiber to CA3 synapse / regulation of membrane potential / SNARE binding / excitatory postsynaptic potential / synaptic transmission, glutamatergic / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / PDZ domain binding / postsynaptic density membrane / regulation of long-term neuronal synaptic plasticity / modulation of chemical synaptic transmission / terminal bouton / intracellular calcium ion homeostasis / positive regulation of neuron apoptotic process / presynaptic membrane / scaffold protein binding / chemical synaptic transmission / perikaryon / postsynaptic membrane / neuron apoptotic process / negative regulation of neuron apoptotic process / postsynaptic density / axon / neuronal cell body / glutamatergic synapse / ubiquitin protein ligase binding / dendrite / synapse / identical protein binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  | |||||||||
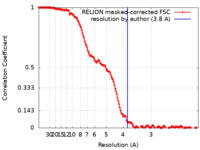
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Bogdanovic N / Tajima N | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Structural basis for kainate receptor activation by a partial agonist Authors: Bogdanovic N / Tajima N | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29927.map.gz emd_29927.map.gz | 225.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29927-v30.xml emd-29927-v30.xml emd-29927.xml emd-29927.xml | 18.1 KB 18.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_29927_fsc.xml emd_29927_fsc.xml | 14.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_29927.png emd_29927.png | 80.5 KB | ||
| Filedesc metadata |  emd-29927.cif.gz emd-29927.cif.gz | 6.7 KB | ||
| Others |  emd_29927_half_map_1.map.gz emd_29927_half_map_1.map.gz emd_29927_half_map_2.map.gz emd_29927_half_map_2.map.gz | 225.5 MB 226.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29927 http://ftp.pdbj.org/pub/emdb/structures/EMD-29927 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29927 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29927 | HTTPS FTP |
-Validation report
| Summary document |  emd_29927_validation.pdf.gz emd_29927_validation.pdf.gz | 935.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_29927_full_validation.pdf.gz emd_29927_full_validation.pdf.gz | 934.7 KB | Display | |
| Data in XML |  emd_29927_validation.xml.gz emd_29927_validation.xml.gz | 22.4 KB | Display | |
| Data in CIF |  emd_29927_validation.cif.gz emd_29927_validation.cif.gz | 29.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29927 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29927 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29927 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29927 | HTTPS FTP |
-Related structure data
| Related structure data |  8gc3MC  8gc2C  8gc4C  8gc5C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29927.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29927.map.gz / Format: CCP4 / Size: 282.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Domote-bound GluK2 kainate receptors in partially-open conformation 2 | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Half Map 1
| File | emd_29927_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 1 | ||||||||||||
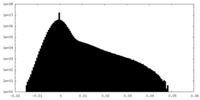
| Projections & Slices |
| ||||||||||||
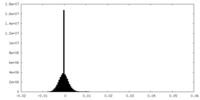
| Density Histograms |
-Half map: Half Map 2
| File | emd_29927_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half Map 2 | ||||||||||||
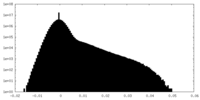
| Projections & Slices |
| ||||||||||||
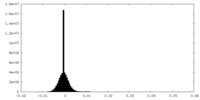
| Density Histograms |
- Sample components
Sample components
-Entire : Homotetrameric GluK2 bound with DOQ state 1
| Entire | Name: Homotetrameric GluK2 bound with DOQ state 1 |
|---|---|
| Components |
|
-Supramolecule #1: Homotetrameric GluK2 bound with DOQ state 1
| Supramolecule | Name: Homotetrameric GluK2 bound with DOQ state 1 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 Details: Tetramer isolated and dyalised extensively. The DOQ was applied prior to grid freezing. |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Glutamate receptor ionotropic, kainate 2
| Macromolecule | Name: Glutamate receptor ionotropic, kainate 2 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 98.883469 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: HVLRFGGIFE YVESGPMGAE ELAFRFAVNT INRNRTLLPN TTLTYDTQKI NLYDSFEASK KACDQLSLGV AAIFGPSHSS SANAVQSIC NALGVPHIQT RWKHQVSDNK DSFYVSLYPD FSSLSRAILD LVQFFKWKTV TVVYDDSTGL IRLQELIKAP S RYNLRLKI ...String: HVLRFGGIFE YVESGPMGAE ELAFRFAVNT INRNRTLLPN TTLTYDTQKI NLYDSFEASK KACDQLSLGV AAIFGPSHSS SANAVQSIC NALGVPHIQT RWKHQVSDNK DSFYVSLYPD FSSLSRAILD LVQFFKWKTV TVVYDDSTGL IRLQELIKAP S RYNLRLKI RQLPADTKDA KPLLKEMKRG KEFHVIFDCS HEMAAGILKQ ALAMGMMTEY YHYIFTTLDL FALDVEPYRY SG VNMTGFR ILNTENTQVS SIIEKWSMER LQAPPKPDSG LLDGFMTTDA ALMYDAVHVV SVAVQQFPQM TVSSLQCNRH KPW RFGTRF MSLIKEAHWE GLTGRITFNK TNGLRTDFDL DVISLKEEGL EKIGTWDPAS GLNMTESQKG KPANITDSLS NRSL IVTTI LEEPYVLFKK SDKPLYGNDR FEGYCIDLLR ELSTILGFTY EIRLVEDGKY GAQDDVNGQW NGMVRELIDH KADLA VAPL AITYVREKVI DFSKPFMTLG ISILYRKPNG TNPGVFSFLN PLSPDIWMYV LLAYLGVSVV LFVIARFSPY EWYNPH PSN PDSDVVENNF TLLNSFWFGV GALMQQGSEL MPKALSTRIV GGIWWFFTLI IISSYTANLA AFLTVERMES PIDSADD LA KQTKIEYGAV EDGATMTFFK KSKISTYDKM WAFMSSRRQS VLVKSNEEGI QRVLTSDYAF LMESTTIEFV TQRNCNLT Q IGGLIDSKGY GVGTPMGSPY RDKITIAILQ LQEEGKLHMM KEKWWRGNGC PEEESKEASA LGVQNIGGIF IVLAAGLVL SVFVAVGEFL YKSKKNAQLE KRSFCSAMVE ELRMSLKCQR RLKHKPQAPV IVKTEEVINM HTFNDRRLPG KETMA UniProtKB: Glutamate receptor ionotropic, kainate 2 |
-Macromolecule #4: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 4 / Number of copies: 6 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #5: (2S,3S,4S)-2-CARBOXY-4-[(1Z,3E,5R)-5-CARBOXY-1-METHYL-1,3-HEXADIE...
| Macromolecule | Name: (2S,3S,4S)-2-CARBOXY-4-[(1Z,3E,5R)-5-CARBOXY-1-METHYL-1,3-HEXADIENYL]-3-PYRROLIDINEACETIC ACID type: ligand / ID: 5 / Number of copies: 4 / Formula: DOQ |
|---|---|
| Molecular weight | Theoretical: 311.33 Da |
| Chemical component information |  ChemComp-DOQ: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 3.5 mg/mL |
|---|---|
| Buffer | pH: 8 / Component - Concentration: 150.0 mmol / Component - Formula: NaCl / Component - Name: sodium chloride / Details: 50 mM Tris/HCl 150 mM NaCl 1mM DDM pH 8.0 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV |
| Details | The sample was isolated from SEC and concentrated. Monodispersity of the final sample determined by the analytical HPLC, Superose 6 increase column |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 12486 / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: OTHER / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 81000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: RIGID BODY FIT / Overall B value: 70 / Target criteria: 0.85 |
|---|---|
| Output model |  PDB-8gc3: |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)