+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23157 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of a CRISPR-Cas Binary Complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | CRISPR CAS / RNA BINDING PROTEIN / RNA BINDING PROTEIN-RNA complex | |||||||||
| Biological species | unidentified (others) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.9 Å | |||||||||
 Authors Authors | Chang L / Li Z | |||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2021 Journal: Nucleic Acids Res / Year: 2021Title: Structural basis for substrate recognition and cleavage by the dimerization-dependent CRISPR-Cas12f nuclease. Authors: Renjian Xiao / Zhuang Li / Shukun Wang / Ruijie Han / Leifu Chang /  Abstract: Cas12f, also known as Cas14, is an exceptionally small type V-F CRISPR-Cas nuclease that is roughly half the size of comparable nucleases of this type. To reveal the mechanisms underlying substrate ...Cas12f, also known as Cas14, is an exceptionally small type V-F CRISPR-Cas nuclease that is roughly half the size of comparable nucleases of this type. To reveal the mechanisms underlying substrate recognition and cleavage, we determined the cryo-EM structures of the Cas12f-sgRNA-target DNA and Cas12f-sgRNA complexes at 3.1 and 3.9 Å, respectively. An asymmetric Cas12f dimer is bound to one sgRNA for recognition and cleavage of dsDNA substrate with a T-rich PAM sequence. Despite its dimerization, Cas12f adopts a conserved activation mechanism among the type V nucleases which requires coordinated conformational changes induced by the formation of the crRNA-target DNA heteroduplex, including the close-to-open transition in the lid motif of the RuvC domain. Only one RuvC domain in the Cas12f dimer is activated by substrate recognition, and the substrate bound to the activated RuvC domain is captured in the structure. Structure-assisted truncated sgRNA, which is less than half the length of the original sgRNA, is still active for target DNA cleavage. Our results expand our understanding of the diverse type V CRISPR-Cas nucleases and facilitate potential genome editing applications using the miniature Cas12f. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23157.map.gz emd_23157.map.gz | 4.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23157-v30.xml emd-23157-v30.xml emd-23157.xml emd-23157.xml | 10.8 KB 10.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_23157.png emd_23157.png | 67.3 KB | ||
| Filedesc metadata |  emd-23157.cif.gz emd-23157.cif.gz | 5.4 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23157 http://ftp.pdbj.org/pub/emdb/structures/EMD-23157 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23157 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23157 | HTTPS FTP |
-Validation report
| Summary document |  emd_23157_validation.pdf.gz emd_23157_validation.pdf.gz | 349.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_23157_full_validation.pdf.gz emd_23157_full_validation.pdf.gz | 348.9 KB | Display | |
| Data in XML |  emd_23157_validation.xml.gz emd_23157_validation.xml.gz | 6 KB | Display | |
| Data in CIF |  emd_23157_validation.cif.gz emd_23157_validation.cif.gz | 6.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23157 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23157 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23157 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23157 | HTTPS FTP |
-Related structure data
| Related structure data |  7l48MC  7l49C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_23157.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23157.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
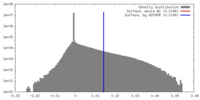
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Cryo-EM structure of a CRISPR-Cas Ternary Complex
| Entire | Name: Cryo-EM structure of a CRISPR-Cas Ternary Complex |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of a CRISPR-Cas Ternary Complex
| Supramolecule | Name: Cryo-EM structure of a CRISPR-Cas Ternary Complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|
-Supramolecule #2: Cas12f
| Supramolecule | Name: Cas12f / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism: unidentified (others) |
-Supramolecule #3: sgRNA
| Supramolecule | Name: sgRNA / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism: unidentified (others) |
-Macromolecule #1: Cas12f
| Macromolecule | Name: Cas12f / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: unidentified (others) |
| Molecular weight | Theoretical: 61.677434 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAKNTITKTL KLRIVRPYNS AEVEKIVADE KNNREKIALE KNKDKVKEAC SKHLKVAAYC TTQVERNACL FCKARKLDDK FYQKLRGQF PDAVFWQEIS EIFRQLQKQA AEIYNQSLIE LYYEIFIKGK GIANASSVEH YLSDVCYTRA AELFKNAAIA S GLRSKIKS ...String: MAKNTITKTL KLRIVRPYNS AEVEKIVADE KNNREKIALE KNKDKVKEAC SKHLKVAAYC TTQVERNACL FCKARKLDDK FYQKLRGQF PDAVFWQEIS EIFRQLQKQA AEIYNQSLIE LYYEIFIKGK GIANASSVEH YLSDVCYTRA AELFKNAAIA S GLRSKIKS NFRLKELKNM KSGLPTTKSD NFPIPLVKQK GGQYTGFEIS NHNSDFIIKI PFGRWQVKKE IDKYRPWEKF DF EQVQKSP KPISLLLSTQ RRKRNKGWSK DEGTEAEIKK VMNGDYQTSY IEVKRGSKIC EKSAWMLNLS IDVPKIDKGV DPS IIGGID VGVKSPLVCA INNAFSRYSI SDNDLFHFNK KMFARRRILL KKNRHKRAGH GAKNKLKPIT ILTEKSERFR KKLI ERWAC EIADFFIKNK VGTVQMENLE SMKRKEDSYF NIRLRGFWPY AEMQNKIEFK LKQYGIEIRK VAPNNTSKTC SKCGH LNNY FNFEYRKKNK FPHFKCEKCN FKENADYNAA LNISNPKLKS TKEEP |
-Macromolecule #2: sgRNA
| Macromolecule | Name: sgRNA / type: rna / ID: 2 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism: unidentified (others) |
| Molecular weight | Theoretical: 72.442969 KDa |
| Sequence | String: GGGCUUCACU GAUAAAGUGG AGAACCGCUU CACCAAAAGC UGUCCCUUAG GGGAUUAGAA CUUGAGUGAA GGUGGGCUGC UUGCAUCAG CCUAAUGUCG AGAAGUGCUU UCUUCGGAAA GUAACCCUCG AAACAAAUUC AUUUUUCCUC UCCAAUUCUG C ACAAGAAA ...String: GGGCUUCACU GAUAAAGUGG AGAACCGCUU CACCAAAAGC UGUCCCUUAG GGGAUUAGAA CUUGAGUGAA GGUGGGCUGC UUGCAUCAG CCUAAUGUCG AGAAGUGCUU UCUUCGGAAA GUAACCCUCG AAACAAAUUC AUUUUUCCUC UCCAAUUCUG C ACAAGAAA GUUGCAGAAC CCGAAUAGAC GAAUGAAGGA AUGCAACAGU UGACCCAACG UCGCCGG |
-Macromolecule #3: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 3 / Number of copies: 4 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 54.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: OTHER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: INSILICO MODEL |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.9 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 154090 |
| Initial angle assignment | Type: RANDOM ASSIGNMENT |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)