[English] 日本語
 Yorodumi
Yorodumi- EMDB-22560: Polyclonal immune complex of Fab binding the receptor binding sit... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-22560 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
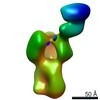
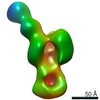
| Title | Polyclonal immune complex of Fab binding the receptor binding site region of H5 HA from serum of subject 43 at day 100 | |||||||||
 Map data Map data | Polyclonal immune complex of Fab binding the receptor binding site region of H5 HA from serum of subject 43 at day 100 | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / negative staining / Resolution: 20.0 Å | |||||||||
 Authors Authors | Ward A / Han J / Richey ST | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2021 Journal: Cell Rep / Year: 2021Title: Polyclonal epitope mapping reveals temporal dynamics and diversity of human antibody responses to H5N1 vaccination. Authors: Julianna Han / Aaron J Schmitz / Sara T Richey / Ya-Nan Dai / Hannah L Turner / Bassem M Mohammed / Daved H Fremont / Ali H Ellebedy / Andrew B Ward /  Abstract: Novel influenza A virus (IAV) strains elicit recall immune responses to conserved epitopes, making them favorable antigenic choices for universal influenza virus vaccines. Evaluating these immunogens ...Novel influenza A virus (IAV) strains elicit recall immune responses to conserved epitopes, making them favorable antigenic choices for universal influenza virus vaccines. Evaluating these immunogens requires a thorough understanding of the antigenic sites targeted by the polyclonal antibody (pAb) response, which single-particle electron microscopy (EM) can sensitively detect. In this study, we employ EM polyclonal epitope mapping (EMPEM) to extensively characterize the pAb response to hemagglutinin (HA) after H5N1 immunization in humans. Cross-reactive pAbs originating from memory B cells immediately bound the stem of HA and persisted for more than a year after vaccination. In contrast, de novo pAb responses to multiple sites on the head of HA, targeting previously determined key neutralizing sites on H5 HA, expanded after the second immunization and waned quickly. Thus, EMPEM provides a robust tool for comprehensively tracking the specificity and durability of immune responses elicited by novel universal influenza vaccine candidates. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_22560.map.gz emd_22560.map.gz | 11.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-22560-v30.xml emd-22560-v30.xml emd-22560.xml emd-22560.xml | 15.8 KB 15.8 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_22560.png emd_22560.png | 37 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-22560 http://ftp.pdbj.org/pub/emdb/structures/EMD-22560 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22560 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-22560 | HTTPS FTP |
-Validation report
| Summary document |  emd_22560_validation.pdf.gz emd_22560_validation.pdf.gz | 298.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_22560_full_validation.pdf.gz emd_22560_full_validation.pdf.gz | 298 KB | Display | |
| Data in XML |  emd_22560_validation.xml.gz emd_22560_validation.xml.gz | 5.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22560 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22560 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22560 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-22560 | HTTPS FTP |
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_22560.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_22560.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Polyclonal immune complex of Fab binding the receptor binding site region of H5 HA from serum of subject 43 at day 100 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.98 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Polyclonal immune complex of Fab binding the receptor binding sit...
| Entire | Name: Polyclonal immune complex of Fab binding the receptor binding site region of H5 HA from serum of subject 43 at day 100 |
|---|---|
| Components |
|
-Supramolecule #1: Polyclonal immune complex of Fab binding the receptor binding sit...
| Supramolecule | Name: Polyclonal immune complex of Fab binding the receptor binding site region of H5 HA from serum of subject 43 at day 100 type: complex / ID: 1 / Parent: 0 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Location in cell: PBMC Homo sapiens (human) / Location in cell: PBMC |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Staining | Type: NEGATIVE / Material: 2% w/v uranyl formate |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Average electron dose: 25.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: DARK FIELD |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 20.0 Å / Resolution method: FSC 0.5 CUT-OFF / Number images used: 5009 |
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
 Movie
Movie Controller
Controller











































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






















